
Batteries for hybrid and electric vehicles
Content
 In our previous article, we discussed the battery as a source of electricity, needed primarily to start a car, as well as for the relatively short-term operation of electrical equipment. However, completely different requirements are imposed on the properties of batteries used in the field of propelling large mobile devices, in our case, hybrid vehicles and electric vehicles. A much larger amount of stored energy is required to power a vehicle and needs to be stored somewhere. In a classic car with an internal combustion engine, it is stored in the tank in the form of gasoline, diesel or LPG. In the case of an electric vehicle or a hybrid vehicle, it is stored in batteries, which can be described as the main problem with an electric vehicle.
In our previous article, we discussed the battery as a source of electricity, needed primarily to start a car, as well as for the relatively short-term operation of electrical equipment. However, completely different requirements are imposed on the properties of batteries used in the field of propelling large mobile devices, in our case, hybrid vehicles and electric vehicles. A much larger amount of stored energy is required to power a vehicle and needs to be stored somewhere. In a classic car with an internal combustion engine, it is stored in the tank in the form of gasoline, diesel or LPG. In the case of an electric vehicle or a hybrid vehicle, it is stored in batteries, which can be described as the main problem with an electric vehicle.
Current accumulators can store little energy, while they are rather bulky, heavy, and at the same time, for their maximum replenishment, it takes several hours (usually 8 or more). In contrast, conventional vehicles with internal combustion engines can store a large amount of energy compared to batteries in a small case, provided that it only takes a minute, maybe two, to recharge. Unfortunately, the problem of storing electricity has plagued electric vehicles since their inception, and despite undeniable progress, their energy density required to power a vehicle is still very low. In the next lines, saving email We will discuss energy in more detail and try to bring closer the real reality of cars with pure electric or hybrid drive. There are many myths around these "electronic cars", so it doesn't hurt to take a closer look at the advantages or disadvantages of such drives.
Unfortunately, the figures given by the manufacturers are also very doubtful and are rather theoretical. For example, the Kia Venga contains an electric motor with a power of 80 kW and a torque of 280 Nm. Power is supplied by lithium-ion batteries with a capacity of 24 kWh, the estimated range of Kia Vengy EV according to the manufacturer is 180 km. The capacity of the batteries tells us that, fully charged, they can provide an engine consumption of 24 kW, or feed a consumption of 48 kW in half an hour, etc. A simple recalculation, and we will not be able to drive 180 km. If we wanted to think about such a range, then we would have to drive an average of 60 km / h for about 3 hours, and the engine power would be only a tenth of the nominal value, i.e. 8 kW. In other words, with a really careful (careful) ride, where you will almost certainly use the brake in work, such a ride is theoretically possible. Of course, we do not consider the inclusion of various electrical accessories. Everyone can already imagine what a self-denial compared to a classic car. At the same time, you pour 40 liters of diesel fuel into the classic Venga and drive hundreds and hundreds of kilometers without restrictions. Why is it so? Let's try to compare how much of this energy and how much weight a classic car can hold in the tank, and how much an electric car can hold in batteries - read more here HERE.
A few facts from chemistry and physics
- calorific value of gasoline: 42,7 MJ / kg,
- calorific value of diesel fuel: 41,9 MJ / kg,
- gasoline density: 725 kg / m3,
- oil density: 840 kg / m3,
- Joule (J) = [kg * m2 / s2],
- Watt (W) = [J / s],
- 1 MJ = 0,2778 kWh.
Energy is the ability to do work, measured in joules (J), kilowatt hours (kWh). Work (mechanical) is manifested by a change in energy during the movement of the body, has the same units as energy. Power expresses the amount of work done per unit of time, the base unit being the watt (W).
| Energy resource | Calorific value / kg density | Calorific value / l Energy / l | Energy / kg |
|---|---|---|---|
| Petrol | 42,7 MJ / kg 725 kg / m3 | 30,96 MJ / l 8,60 kWh / l | 11,86 kWh / kg |
| Oil | 41,9 MJ / kg 840 kg / m3 | 35,20 MJ / l 9,78 kWh / l | 11,64 kWh / kg |
| Li-ion battery (Audi R8 e-tron) | 42 kWh 470 kg | 0,0893 kWh / kg |
From the above it is clear that, for example, with a calorific value of 42,7 MJ / kg and a density of 725 kg / m3, gasoline offers an energy of 8,60 kWh per liter or 11,86 kWh per kilogram. If we build the current batteries that are now installed in electric vehicles, for example, lithium-ion, their capacity is less than 0,1 kWh per kilogram (for simplicity, we will consider 0,1 kWh). Conventional fuels provide over a hundred times more energy for the same weight. You will understand that this is a huge difference. If we break it down into small ones, for example, a Chevrolet Cruze with a 31 kWh battery carries energy that can fit in less than 2,6 kg of gasoline or, if you want, about 3,5 liters of gasoline.
You can tell how it is possible that an electric car will start at all, and not that it will still have more than 100 km of energy. The reason is simple. The electric motor is much more efficient in terms of converting stored energy into mechanical energy. Typically, it should have an efficiency of 90%, while the efficiency of an internal combustion engine is about 30% for a gasoline engine and 35% for a diesel engine. Therefore, to provide the same power to the electric motor, it is enough with a much lower energy reserve.
Ease of use of individual drives
After evaluating the simplified calculation, it is assumed that we can obtain approximately 2,58 kWh of mechanical energy from a liter of gasoline, 3,42 kWh from a liter of diesel fuel, and 0,09 kWh from a kilogram of a lithium-ion battery. So the difference is not more than a hundredfold, but only about thirty times. This is the best number, but still not really pink. For example, consider the sporty Audi R8. Its fully charged batteries, weighing 470 kg, have an energy equivalent of 16,3 liters of petrol or just 12,3 liters of diesel fuel. Or, if we had an Audi A4 3,0 TDI with a tank capacity of 62 liters of diesel fuel and we wanted to have the same range on a pure battery drive, we would need approximately 2350 kg of batteries. So far, this fact does not give the electric car a very bright future. However, there is no need to throw a shotgun at the rye, as the pressure to develop such "e-cars" will be taken off by the ruthless green lobby, so whether automakers like it or not, they must produce something "green." “. A definite replacement for a purely electric drive is the so-called hybrids, which combine an internal combustion engine with an electric motor. Currently the best known are, for example, the Toyota Prius (Auris HSD with the same hybrid technology) or the Honda Inside. However, their purely electrical range is still laughable. In the first case, about 2 km (in the latest version of Plug In it is increased “to” 20 km), and in the second, Honda does not even knock on a purely electric drive. So far, the resulting effectiveness in practice is not as miraculous as mass advertising suggests. Reality has shown that they can color them with any blue movement (economy) mostly with conventional technology. The advantage of the hybrid power plant lies mainly in fuel economy when driving in the city. Audi recently said that currently it is only necessary to reduce body weight to achieve, on average, the same fuel economy that some brands achieve by installing a hybrid system in a car. New models of some cars also prove that this is not a scream into the dark. For example, the recently introduced seventh generation Volkswagen Golf uses lighter components to learn from and in practice actually uses less fuel than before. Japanese automaker Mazda has taken a similar direction. Despite these claims, the development of a "long-range" hybrid drive continues. As an example, I will mention the Opel Ampera and, paradoxically, the model from the Audi A1 e-tron.
| Energy resource | Engine efficiency | Effective energy / l | Effective energy / kg |
|---|---|---|---|
| Petrol | 0,30 | 2,58 kWh / l | 3,56 kWh / kg |
| Oil | 0,35 | 3,42 kWh / l | 4,07 kWh / kg |
| Lithium ion batteries | 0,90 | - | OK. 0,1 kWh / kg |
Opel Ampera
Although the Opel Ampera is often presented as an electric car, it is actually a hybrid car. In addition to the electric motor, the Ampere also uses a 1,4-liter 63 kW internal combustion engine. However, this gasoline engine does not directly drive the wheels, but acts as a generator in case the batteries run out of electricity. energy. The electrical part is represented by an electric motor with an output of 111 kW (150 hp) and a torque of 370 Nm. The power supply is powered by 220 T-shaped lithium cells. They have a total power of 16 kWh and weigh 180 kg. This electric car can travel 40-80 km on a purely electric drive. This distance is often sufficient for all-day city driving and significantly reduces operating costs as city traffic requires significant fuel consumption in the case of combustion engines. The batteries can also be recharged from a standard outlet, and when combined with the internal combustion engine, the Ampera's range extends to a very respectable five hundred kilometers.
Audi e electron A1
Audi, which prefers a classic drive with more advanced technology than a technically very demanding hybrid drive, introduced an interesting A1 e-tron hybrid car more than two years ago. Lithium-ion batteries with a capacity of 12 kWh and a weight of 150 kg are charged by a Wankel engine as part of a generator that uses the energy in the form of gasoline stored in a 254-liter tank. The engine has a volume of 15 cubic meters. cm and generates 45 kW / h el. energy. The electric motor has a power of 75 kW and can produce up to 0 kW of power in a short time. Acceleration from 100 to 10 is about 130 seconds and a top speed of about 50 km / h. The car can travel about 12 km around the city on a purely electric drive. After the depletion of e. the energy is discreetly activated by the rotary internal combustion engine and recharges the electricity. energy for batteries. The total range with fully charged batteries and 250 liters of petrol is about 1,9 km with an average consumption of 100 liters per 1450 km. The operating weight of the vehicle is 12 kg. Let's take a look at a simple conversion to see in direct comparison how much energy is hidden in a 30 liter tank. Assuming a modern Wankel engine efficiency of 70%, then 9 kg of it, together with 12 kg (31 L) of gasoline, is equivalent to 79 kWh of energy stored in batteries. So 387,5 kg of engine and tank = 1 kg of batteries (calculated in Audi A9 e-Tron weights). If we wanted to increase the fuel tank by 62 liters, we would already have XNUMX kWh of energy available to power the car. So we could continue. But he must have one catch. It will no longer be a "green" car. So even here it is clearly seen that the electric drive is significantly limited by the power density of the energy stored in the batteries.
In particular, the higher price, as well as the high weight, have led to the fact that the hybrid drive in Audi has gradually faded into the background. However, this does not mean that the development of hybrid cars and electric vehicles at Audi has completely depreciated. Information about the new version of the A1 e-tron model has appeared recently. Compared to the previous one, the rotary engine/generator has been replaced by a 1,5 kW 94-litre three-cylinder turbocharged engine. The use of the classic internal combustion unit was forced by Audi mainly due to the difficulties associated with this transmission, and the new three-cylinder engine is designed not only to charge the batteries, but also work directly with the drive wheels. The Sanyo batteries have an identical output of 12kWh, and the range of the purely electric drive has been slightly increased to approximately 80km. Audi says the upgraded A1 e-tron should average one liter per hundred kilometers. Unfortunately, this expense has one snag. For hybrid vehicles with extended pure electric range. drive uses an interesting technique for calculating the final flow rate. So-called consumption is ignored. refueling from the battery charging network, as well as the final consumption l / 100 km, only takes into account the consumption of gasoline for the last 20 km of driving, when there is electricity. battery charge. By a very simple calculation, we can calculate this if the batteries were suitably discharged. we drove after the power went out. energy from purely gasoline batteries, as a result, consumption will increase five times, that is, 5 liters of gasoline per 100 km.
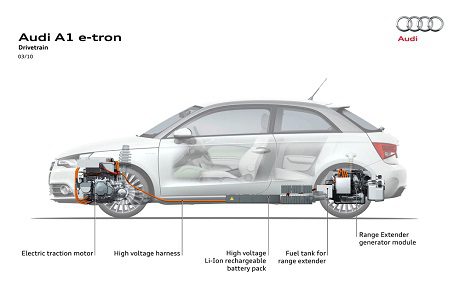
Audi A1 e-tron II. generation

Electricity storage problems
The issue of energy storage is as old as electrical engineering itself. The first sources of electricity were galvanic cells. After a short time, the possibility of a reversible process of accumulation of electricity in galvanic secondary cells - batteries was discovered. The first used batteries were lead batteries, after a short time nickel-iron and a little later nickel-cadmium, and their practical use lasted more than a hundred years. It should also be added that, despite intensive worldwide research in this area, their basic design has not changed much. Using new manufacturing technologies, improving the properties of base materials and using new materials for cell and vessel separators, it was possible to slightly reduce the specific gravity, reduce the self-discharge of the cells, and increase the comfort and safety of the operator, but that's about it. The most significant drawback, ie. A very unfavorable ratio of the amount of stored energy to the weight and volume of the batteries remained. Therefore, these batteries were used mainly in static applications (backup power supplies in case the main power supply fails, etc.). Batteries were used as a source of energy for traction systems, especially on railways (transport carts), where heavy weight and significant dimensions also did not interfere too much.
Energy storage progress
However, the need to develop cells with small capacities and dimensions in ampere hours has increased. Thus, alkaline primary cells and sealed versions of nickel-cadmium (NiCd) and then nickel-metal hydride (NiMH) batteries were formed. For the encapsulation of the cells, the same sleeve shapes and sizes were chosen as for the hitherto conventional primary zinc chloride cells. In particular, the achieved parameters of nickel-metal hydride batteries make it possible to use them, in particular, in mobile phones, laptops, manual drives of tools, etc. The manufacturing technology of these cells differs from the technologies used for cells with a large capacity in ampere-hours. The lamellar arrangement of the large cell electrode system is replaced by the technology of converting the electrode system, including separators, into a cylindrical coil, which is inserted and contacted with regular shaped cells in sizes AAA, AA, C and D, resp. multiples of their size. For some special applications, special flat cells are produced.
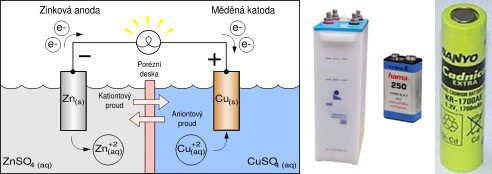
The advantage of hermetic cells with spiral electrodes is several times greater ability to charge and discharge with high currents and the ratio of relative energy density to cell weight and volume compared to the classical large cell design. The disadvantage is more self-discharge and fewer work cycles. The maximum capacity of a single NiMH cell is approximately 10 Ah. But, as with other larger diameter cylinders, they do not allow charging too high currents due to problematic heat dissipation, which greatly reduces the use in electric vehicles, and therefore this source is only used as an auxiliary battery in a hybrid system (Toyota Prius 1,3 .XNUMX kWh).

A significant advance in the field of energy storage has been the development of safe lithium batteries. Lithium is an element with a high electrochemical potential value, but it is also extremely reactive in an oxidative sense, which also causes problems when using lithium metal in practice. When lithium comes into contact with atmospheric oxygen, combustion occurs, which, depending on the properties of the environment, can have the character of an explosion. This unpleasant property can be eliminated either by carefully protecting the surface, or by using less active lithium compounds. Currently, the most common lithium-ion and lithium-polymer batteries with a capacity of 2 to 4 Ah in ampere-hours. Their use is similar to that of NiMh, and at an average discharge voltage of 3,2 V, 6 to 13 Wh of energy is available. Compared to nickel-metal hydride batteries, lithium batteries can store two to four times more energy for the same volume. Lithium-ion (polymer) batteries have an electrolyte in gel or solid form and can be manufactured in flat cells as thin as a few tenths of a millimeter in virtually any shape to suit the needs of the respective application.
The electric drive in a passenger car can be made as the main and only one (electric car) or combined, where the electric drive can be both the dominant and auxiliary source of traction (hybrid drive). Depending on the variant used, the energy requirements for the operation of the vehicle and therefore the capacity of the batteries differ. In electric vehicles, the battery capacity is between 25 and 50 kWh, and with a hybrid drive, it is naturally lower and ranges from 1 to 10 kWh. From the given values it can be seen that at a voltage of one (lithium) cell of 3,6 V, it is necessary to connect the cells in series. In order to reduce losses in distribution conductors, inverters and motor windings, it is recommended to select a voltage higher than usual in the on-board network (12 V) for drives - commonly used values are from 250 to 500 V. From today, Lithium cells are obviously the most suitable type. Admittedly, they are still very expensive, especially when compared to lead-acid batteries. However, they are much more difficult.
The nominal voltage of conventional lithium battery cells is 3,6 V. This value is different from conventional nickel-metal hydride cells, respectively. NiCd, which have a nominal voltage of 1,2 V (or lead - 2 V), which, if used in practice, does not allow interchangeability of both types. The charging of these lithium batteries is characterized by the need to very accurately maintain the value of the maximum charging voltage, which requires a special type of charger and, in particular, does not allow the use of charging systems designed for other types of cells.
Main characteristics of lithium batteries
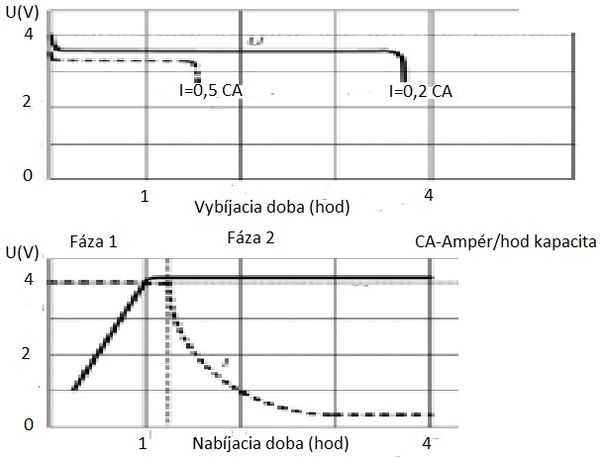
The main characteristics of batteries for electric vehicles and hybrids can be considered their charging and discharging characteristics.
Charging characteristic
The charging process requires regulation of the charging current, the control of the cell voltage and the control of the current temperature cannot be skipped. For lithium cells in use today that use LiCoO2 as the cathode electrode, the maximum charging voltage limit is 4,20 to 4,22 V per cell. Exceeding this value leads to damage to the properties of the cell and, conversely, failure to reach this value means not using the nominal cell capacity. For charging, the usual IU characteristic is used, that is, in the first phase it is charged with constant current until a voltage of 4,20 V / cell is reached. The charging current is limited to the maximum permissible value specified by the cell manufacturer, respectively. charger options. The charging time at the first stage varies from several tens of minutes to several hours, depending on the magnitude of the charging current. Cell voltage gradually increases up to max. values of 4,2 V. As already mentioned, this voltage should not be exceeded due to the risk of damage to the cell. In the first phase of charging, 70 to 80% of the energy is stored in the cells, in the second phase the rest. In the second phase, the charging voltage is maintained at the maximum permissible value, and the charging current gradually decreases. Charging is complete when the current has dropped to about 2–3% of the cell's rated discharge current. Since the maximum value of the charging currents in the case of smaller cells is also several times higher than the discharge current, a significant part of the electricity can be saved in the first charging phase. energy in a relatively very short time (approximately ½ and 1 hour). Thus, in the event of an emergency, it is possible to charge the batteries of an electric vehicle to a sufficient capacity in a relatively short time. Even in the case of lithium cells, the accumulated electricity decreases after a certain period of storage. However, this only happens after about 3 months of downtime.
Discharge characteristics
The voltage first drops rapidly to 3,6–3,0 V (depending on the magnitude of the discharge current) and remains almost constant throughout the entire discharge. After the exhaustion of the supply of e-mail. the energy also lowers the cell voltage very quickly. Therefore, the discharge must be completed no later than the manufacturer's specified discharge voltage of 2,7 to 3,0 V.
Otherwise, the structure of the product may be damaged. The unloading process is relatively easy to control. It is limited only by the value of the current and stops when the value of the final discharge voltage is reached. The only problem is that the properties of individual cells in a sequential arrangement are never the same. Therefore, care must be taken to ensure that the voltage of any cell does not fall below the final discharge voltage, as this can damage it and thus cause the entire battery to malfunction. The same should be considered when charging the battery.
The aforementioned type of lithium cells with a different cathode material, in which the oxide of cobalt, nickel or manganese is replaced by the phosphide Li3V2 (PO4) 3, eliminates the mentioned risks of damage to the cell due to non-compliance. Cells with a higher capacity. Also declared is their declared service life of about 2 charge cycles (at 000% discharge) and especially the fact that when the cell is completely discharged, it will not be damaged. The advantage is also a higher nominal voltage of about 80 when charging up to 4,2 V.
From the above description, it can be clearly indicated that lithium batteries are currently the only alternative such as storing energy for driving a car compared to the energy stored in fossil fuel in a fuel tank. Any increase in battery specific capacity will increase the competitiveness of this eco-friendly drive. We can only hope that development will not slow down, but, on the contrary, move forward several miles.
Examples of vehicles using hybrid and electric batteries
Toyota Prius is a classic hybrid with a low power reserve on pure electric. drive
The Toyota Prius uses a 1,3 kWh NiMH battery, which is primarily used as a power source for acceleration and allows a separate electric drive to be used for a distance of about 2 km at max. speed of 50 km / h. The Plug-In version already uses lithium-ion batteries with a capacity of 5,4 kWh, which allows you to drive exclusively on an electric drive for a distance of 14-20 km at a maximum speed. speed 100 km / h.
Opel Ampere-hybrid with increased power reserve on pure e-mail. drive
The electric vehicle with an extended range (40-80 km), as Opel calls the four-seater five-door Amper, is powered by an electric motor producing 111 kW (150 hp) and 370 Nm of torque. The power supply is powered by 220 T-shaped lithium cells. They have a total power of 16 kWh and weigh 180 kg. The generator is a 1,4 liter gasoline engine with 63 kW output.
Mitsubishi and MiEV, Citroën C-Zero, Peugeot iOn-clean el. cars
Lithium-ion batteries with a capacity of 16 kWh allow the vehicle to travel up to 150 km without recharging, as measured in accordance with the NEDC (New European Driving Cycle) standard. The high-voltage batteries (330 V) are located inside the floor and are also protected by the cradle frame from damage in the event of an impact. It is a product of Lithium Energy Japan, a joint venture between Mitsubishi and GS Yuasa Corporation. There are 88 articles in total. Electricity for the drive is provided by a 330 V lithium-ion battery, consisting of 88 50 Ah cells with a total capacity of 16 kWh. The battery will be charged from a home outlet for six hours, using an external fast charger (125 A, 400 V), the battery will be charged to 80% in half an hour.

I myself am a big fan of electric vehicles and constantly monitor what is happening in this area, but the reality at the moment is not so optimistic. This is also confirmed by the above information, which shows that the life of both pure electric and hybrid vehicles is not easy, and often only a numbers game pretends to be. Their production is still very demanding and expensive, and their effectiveness is repeatedly debatable. The main disadvantage of electric vehicles (hybrids) is the very low specific capacity of the energy stored in batteries compared to the energy stored in conventional fuels (diesel, gasoline, liquefied petroleum gas, compressed natural gas). To really bring the power of electric vehicles closer to conventional cars, batteries would have to reduce their weight by at least a tenth. This means that the mentioned Audi R8 e-tron had to store 42 kWh not in 470 kg, but in 47 kg. In addition, the charging time would have to be significantly reduced. About an hour at 70-80% capacity is still a lot, and I'm not talking about 6-8 hours on average on a full charge. There is no need to believe the bullshit about zero production of CO2 electric vehicles either. Let us immediately note the fact that The energy in our sockets is also generated by thermal power plants, and they not only produce enough CO2. Not to mention the more complex production of such a car, where the need for CO2 for production is much greater than in a classic one. We must not forget about the number of components containing heavy and toxic materials and their problematic subsequent disposal.
With all the minuses mentioned and not mentioned, an electric car (hybrid) also has undeniable advantages. In urban traffic or over shorter distances, their more economical operation is undeniable, only because of the principle of energy storage (recovery) during braking, when in conventional vehicles it is removed during braking in the form of waste heat into the air, not to mention the possibility a few km drive around the city for cheap recharging from public e-mail. net. If we compare a pure electric car and a classic car, then in a conventional car there is an internal combustion engine, which in itself is a rather complex mechanical element. Its power must be transferred to the wheels in some way, and this is mostly done through a manual or automatic transmission. There is still one or more differentials in the way, sometimes also a driveshaft and a series of axle shafts. Of course, the car also needs to slow down, the engine needs to cool down, and this thermal energy is uselessly lost to the environment as residual heat. An electric car is much more efficient and simpler - (does not apply to a hybrid drive, which is very complicated). The electric car does not contain gearboxes, gearboxes, cardans and half shafts, forget about the engine in front, rear or in the middle. It does not contain a radiator, i.e. coolant and starter. The advantage of an electric car is that it can install motors directly into the wheels. And suddenly you have the perfect ATV that can control each wheel independently of the others. Therefore, with an electric vehicle, it will not be difficult to control only one wheel, and it is also possible to select and control the optimal distribution of power for cornering. Each of the motors can also be a brake, again completely independent of the other wheels, that converts at least some of the kinetic energy back into electrical energy. As a result, conventional brakes will be subjected to much less stress. The engines can produce the maximum available power at almost any time and without delay. Their efficiency in converting energy stored in batteries into kinetic energy is about 90%, which is about three times that of conventional motors. Consequently, they do not generate as much residual heat and do not need to be difficult to cool. All you need for this is good hardware, a control unit and a good programmer.
Suma sumárum. If electric cars or Hybrids are even closer to classic cars with fuel efficient engines, they still have a very difficult and difficult path ahead of them. I just hope this is not confirmed by a number of misleading numbers or. exaggerated pressure from officials. But let's not despair. The development of nanotechnology is really moving by leaps and bounds, and, perhaps, miracles are really in store for us in the near future.
Finally, I will add one more interesting thing. There is already a solar refueling station.

Toyota Industries Corp (TIC) has developed a solar charging station for electric and hybrid vehicles. The station is also connected to the power grid, so the 1,9 kW solar panels are more likely an additional source of energy. Using a self-contained (solar) power source, the charging station can provide a maximum power of 110 VAC / 1,5 kW, when connected to the mains, it offers a maximum of 220 VAC / 3,2 kW.
Unused electricity from solar panels is stored in batteries, which can store 8,4 kWh for later use. It is also possible to supply electricity to the distribution network or supply station accessories. The charging stands used at the station have built-in communication technology capable of identifying vehicles accordingly. their owners using smart cards.
Important terms for batteries
- Power - indicates the amount of electrical charge (amount of energy) stored in the battery. It is specified in ampere hours (Ah) or, in the case of small devices, in milliamp hours (mAh). A 1 Ah (= 1000 mAh) battery is theoretically capable of delivering 1 amp for one hour.
- Internal resistance - indicates the ability of the battery to provide more or less discharge current. For illustration, two canisters can be used, one with a smaller outlet (high internal resistance) and the other with a larger one (low internal resistance). If we decide to empty them, a canister with a smaller drain hole will empty more slowly.
- Battery rated voltage - for nickel-cadmium and nickel-metal hydride batteries, it is 1,2 V, lead 2 V and lithium from 3,6 to 4,2 V. During operation, this voltage varies within 0,8 - 1,5 V for nickel -cadmium and nickel-metal hydride batteries, 1,7 - 2,3 V for lead and 3-4,2 and 3,5-4,9 for lithium.
- Charging current, discharge current – expressed in amperes (A) or milliamps (mA). This is important information for the practical use of the battery in question for a particular device. It also determines the conditions for the correct charging and discharging of the battery so that its capacity is used to the maximum and at the same time not destroyed.
- Charging acc. discharge curve - graphically displays the change in voltage depending on the time when charging or discharging the battery. When a battery is discharged, there is typically a small change in voltage for approximately 90% of the discharge time. Therefore, it is very difficult to determine the current state of the battery from the measured voltage.
- Self-discharge, self-discharge – The battery cannot maintain electricity all the time. energy, since the reaction at the electrodes is a reversible process. A charged battery gradually discharges on its own. This process can take from several weeks to months. In the case of lead-acid batteries, this is 5-20% per month, for nickel-cadmium batteries - about 1% of the electric charge per day, in the case of nickel-metal hydride batteries - about 15-20% per month, and lithium loses about 60%. capacity for three months. Self-discharge depends on the ambient temperature as well as internal resistance (batteries with higher internal resistance discharge less) and of course the design, materials used and workmanship are also important.
- Battery (kits) – Only in exceptional cases are batteries used individually. Usually they are connected in a set, almost always connected in series. The maximum current of such a set is equal to the maximum current of an individual cell, the rated voltage is the sum of the rated voltages of the individual cells.
- Accumulation of batteries. A new or unused battery should be subjected to one, but preferably several (3-5) slow full charge and slow discharge cycles. This slow process sets the battery parameters to the desired level.
- Memory effect – This happens when the battery is charged and discharged to the same level with approximately constant, not too much current, and there should not be a full charge or deep discharge of the cell. This side effect affected NiCd (minimally also NiMH).