What does a catalytic converter do?
The modern car exhaust system is much more advanced than what was available even just a couple of decades ago. Recognizing that the average car is a major source of global pollution, the US government passed the Clean Air Act requiring all cars manufactured after that date to have a working catalytic converter, among other critical components. Your "cat" sits in your car's exhaust system, running quietly and reducing harmful emissions.
What is it to do?
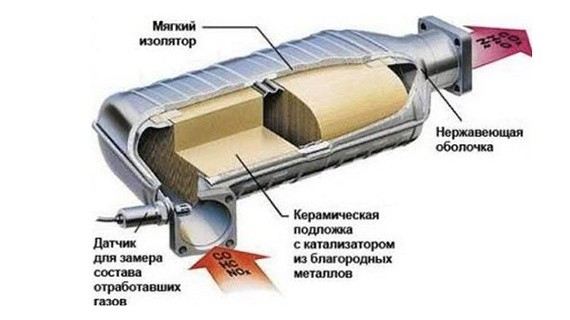
A catalytic converter has one job: to reduce harmful emissions in your car's exhaust to reduce pollution. It uses a catalyst (actually more than one) to convert harmful chemicals such as carbon monoxide, hydrocarbons, and oxides of nitrogen into harmless substances. The catalyst can be one of three metals, or a combination of them:
- Platinum
- Palladium
- Rhodium
Some catalytic converter manufacturers are now adding gold to the mix because it's actually cheaper than the other three metals and can provide better oxidation for some chemicals.
What is oxidation?
Oxidation is used in this sense to mean "burning". Essentially, the catalyst is heated to very high temperatures. These temperatures, combined with the unique properties of the metals used as catalysts, create chemical changes in unwanted substances. By changing the chemical composition, they become harmless.
Carbon monoxide (poisonous) turns into carbon dioxide. Nitrogen oxides are broken down into nitrogen and oxygen, two naturally occurring elements in the atmosphere anyway. Hydrocarbons left over from unburned fuel are converted into water and carbon dioxide.