What is a catalytic converter car?
Content
Catalytic Car Converter
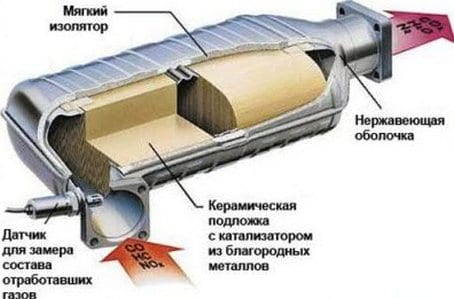
The catalyst in the exhaust system is designed to reduce emissions of harmful substances into the atmosphere. With exhaust gases turning them into harmless components. The catalyst is used on both gasoline and diesel engines. Three-way catalytic converter. Used in gasoline engines. He works on the stoichiometric composition of the mixture, which ensures complete combustion of the fuel. The design of the three-way catalytic converter includes a support block, thermal insulation and housing. The main element of the catalytic converter is the support block, which serves as the basis for the catalysts. The bearing block is made of special refractory ceramics. Structurally, the support block consists of a set of longitudinal cells. Which significantly increases the area of contact with exhaust gases.
Catalytic converter components
Catalytic substances are applied to the surface of the cells. A thin layer that includes three components: platinum, palladium and rhodium. Catalysts accelerate the course of chemical reactions in the catalyst. Platinum and palladium are oxidation catalysts. They contribute to the oxidation of unburned hydrocarbons in water vapor, from carbon monoxide, carbon monoxide to carbon dioxide. Rhodium is a reducing catalyst. This reduces nitrogen oxides to harmless nitrogen. Thus, three catalysts reduce the content of three harmful substances in the exhaust gases. The support block is housed in a metal case. Between them is usually a layer of insulation. In the case of a converter, an oxygen sensor is installed. A prerequisite for starting the catalytic converter is to reach a temperature of 300 ° C. An ideal temperature range is 400 to 800 ° C.
Where to install a car catalytic converter
At this temperature, up to 90% of harmful substances are retained. Temperatures above 800 ° C cause sintering of metal catalysts and cell support block. The catalytic converter is usually installed directly behind the exhaust manifold or in front of the muffler. The first installation of the converter contributes to its rapid heating. But then the device is subjected to high thermal loads. In the latter case, additional measures are necessary so that the catalyst can quickly heat up, which increases the temperature of the exhaust gases. Ignition timing adjustment in the deceleration direction; increase idle speed; variable valve timing; several fuel injections per cycle; air supply to the exhaust system.
What provides oxidation of a diesel engine
A three component catalytic catalyst scheme is used to increase efficiency. It is divided into two parts: the primary converter. Which is located behind the exhaust manifold. The main converter, which is located under the bottom of the car. The diesel engine catalyst oxidizes the individual exhaust components with oxygen. Which is present in sufficient quantity in the exhaust gases of a diesel engine. When passing through a catalytic converter, harmful substances carbon monoxide and hydrocarbons are oxidized to harmless products of carbon dioxide and water vapor. In addition, the catalyst almost completely eliminates the unpleasant odor of diesel exhaust.
Catalytic converter
Oxidation reactions in the catalyst also create unwanted products. Thus, sulfur dioxide is oxidized to sulfur trioxide. This is followed by the formation of sulfuric acid. Sulfuric acid gas combines with water molecules. Which leads to the formation of solid particles - sulfates. They accumulate in the converter and reduce its effectiveness. To remove sulphates from the converter, the engine management system initiates a desulfurization process. In which the catalyst is heated to temperatures above 650°C and is purged with enriched exhaust gases. There is no air, until its complete absence. The diesel engine catalyst is not used to reduce nitrogen oxide emissions in the exhaust. This function in a diesel engine is performed by the system. Exhaust gas recirculation or more advanced selective catalytic converter system.
Questions and answers:
What is the principle behind the work of an exhaust gas catalytic converter? A chemical reaction takes place in the catalyst based on high temperature and contact of nitrogen oxides with precious metals. As a result, harmful substances are neutralized.
What is an exhaust gas converter? This is a small container that sits as close as possible to the engine exhaust manifold. Inside this flask is a porcelain filler with honeycomb cells covered with precious metals.
What is the catalytic converter used for? This element of the exhaust system neutralizes harmful substances in the exhaust gases by converting them into less harmful ones.
Where is the catalytic converter located? Since a chemical reaction must take place in the catalyst based on the high temperature, the exhaust gases must not cool down, so the catalyst is as close to the exhaust system of the internal combustion engine as possible.