Before the triple art, that is, about the discovery of artificial radioactivity
From time to time in the history of physics there are "wonderful" years when the joint efforts of many researchers lead to a series of breakthrough discoveries. So it was with 1820, the year of electricity, 1905, the miraculous year of Einstein's four papers, 1913, the year associated with the study of the structure of the atom, and finally 1932, when a series of technical discoveries and advances in the creation of the nuclear physics.
newlyweds
Irina, the eldest daughter of Marie Skłodowska-Curie and Pierre Curie, was born in Paris in 1897 (1). Until the age of twelve, she was brought up at home, in a small "school" created by eminent scientists for her children, in which there were about ten students. The teachers were: Marie Sklodowska-Curie (physics), Paul Langevin (mathematics), Jean Perrin (chemistry), and the humanities were mainly taught by the mothers of the students. Lessons usually took place in teachers' homes, while children studied physics and chemistry in real laboratories.
Thus, the teaching of physics and chemistry was the acquisition of knowledge through practical actions. Each successful experiment delighted young researchers. These were real experiments that had to be understood and carefully carried out, and the children in Marie Curie's laboratory had to be in exemplary order. Theoretical knowledge also had to be acquired. The method, as the fate of the students of this school, later good and outstanding scientists, proved to be effective.
2. Frederic Joliot (photo by Harcourt)
Moreover, Irena's paternal grandfather, a doctor, devoted a lot of time to his father's orphaned granddaughter, having fun and supplementing her natural science education. In 1914, Irene graduated from the pioneering Collège Sévigné and entered the faculty of mathematics and science at the Sorbonne. This coincided with the start of the First World War. In 1916 she joined her mother and together they organized a radiological service in the French Red Cross. After the war, she received a bachelor's degree. In 1921, her first scientific work was published. He was devoted to the determination of the atomic mass of chlorine from various minerals. In her further activities, she worked closely with her mother, dealing with radioactivity. In her doctoral dissertation, defended in 1925, she studied the alpha particles emitted by polonium.
Frederic Joliot born in 1900 in Paris (2). From the age of eight he attended school in So, lived in a boarding school. At that time, he preferred sports to studies, especially football. He then took turns attending two high schools. Like Irene Curie, he lost his father early. In 1919 he passed the examination at the École de Physique et de Chemie Industrielle de la Ville de Paris (School of Industrial Physics and Industrial Chemistry of the city of Paris). He graduated in 1923. His professor, Paul Langevin, learned of Frederick's abilities and virtues. After 15 months of military service, on the orders of Langevin, he was appointed personal laboratory assistant to Marie Skłodowska-Curie at the Radium Institute with a grant from the Rockefeller Foundation. There he met Irene Curie, and in 1926 the young people got married.
Frederick completed his doctoral dissertation on the electrochemistry of radioactive elements in 1930. A little earlier, he had already focused his interests on his wife's research, and after defending Frederick's doctoral dissertation, they already worked together. One of their first important successes was a preparation of polonium, which is a strong source of alpha particles, i.e. helium nuclei.(24He). They started from an undeniably privileged position, because it was Marie Curie who supplied her daughter with a large portion of polonium. Lew Kowarsky, their later collaborator, described them as follows: Irena was "an excellent technician", "she worked very beautifully and carefully", "she deeply understood what she was doing." Her husband had "a more dazzling, more soaring imagination". "They complemented each other perfectly and knew it." From the point of view of the history of science, the most interesting for them were two years: 1932-34.
They almost discovered the neutron
"Almost" matters a lot. They learned about this sad truth very soon. In 1930 in Berlin, two Germans - Walter Bothe i Hubert Becker - Investigated how light atoms behave when bombarded with alpha particles. Beryllium Shield (49Be) when bombarded with alpha particles emitted extremely penetrating and high-energy radiation. According to the experimenters, this radiation must have been strong electromagnetic radiation.
At this stage, Irena and Frederick dealt with the problem. Their source of alpha particles was the most powerful ever. They used a cloud chamber to observe the reaction products. At the end of January 1932, they publicly announced that it was gamma rays that knocked out high-energy protons from a substance containing hydrogen. They did not yet understand what was in their hands and what was happening.. After reading James Chadwick (3) at Cambridge he immediately set to work, thinking that it was not gamma radiation at all, but neutrons predicted by Rutherford several years in advance. After a series of experiments, he became convinced of the observation of the neutron and found that its mass is similar to that of the proton. On February 17, 1932, he submitted a note to the journal Nature entitled "The Possible Existence of the Neutron."
It was actually a neutron, although Chadwick believed that a neutron was made up of a proton and an electron. Only in 1934 did he understand and prove that the neutron is an elementary particle. Chadwick was awarded the Nobel Prize in Physics in 1935. Despite the realization that they had missed an important discovery, the Joliot-Curies continued their research in this area. They realized that this reaction produced gamma rays in addition to neutrons, so they wrote the nuclear reaction:
, where Ef is the energy of the gamma-quantum. Similar experiments were carried out with 919F.
Missed opening again
A few months before the discovery of the positron, Joliot-Curie had photographs of, among other things, a curved path, as if it were an electron, but twisting in the opposite direction of the electron. The photographs were taken in a fog chamber located in a magnetic field. Based on this, the couple talked about electrons going in two directions, from the source and to the source. In fact, those associated with the direction "toward the source" were positrons, or positive electrons moving away from the source.
Meanwhile, in the United States in the late summer of 1932, Carl David Anderson (4), the son of Swedish immigrants, studied cosmic rays in a cloud chamber under the influence of a magnetic field. Cosmic rays come to Earth from outside. Anderson, to be sure of the direction and movement of the particles, inside the chamber passed the particles through a metal plate, where they lost some of the energy. On August 2, he saw a trail, which he undoubtedly interpreted as a positive electron.
It is worth noting that Dirac had previously predicted the theoretical existence of such a particle. However, Anderson did not follow any theoretical principles in his studies of cosmic rays. In this context, he called his discovery accidental.
Again, Joliot-Curie had to put up with an undeniable profession, but undertook further research in this area. They found that gamma-ray photons can disappear near a heavy nucleus, forming an electron-positron pair, apparently in accordance with Einstein's famous formula E = mc2 and the law of conservation of energy and momentum. Later, Frederick himself proved that there is a process of disappearance of an electron-positron pair, giving rise to two gamma quanta. In addition to positrons from electron-positron pairs, they had positrons from nuclear reactions.
5. Seventh Solvay Conference, 1933
Seated in the front row: Irene Joliot-Curie (second from left),
Maria Skłodowska-Curie (fifth from left), Lise Meitner (second from right).
artificial radioactivity
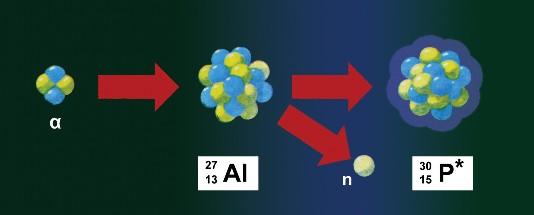
The discovery of artificial radioactivity was not an instantaneous act. In February 1933, by bombarding aluminum, fluorine, and then sodium with alpha particles, Joliot obtained neutrons and unknown isotopes. In July 1933, they announced that, by irradiating aluminum with alpha particles, they observed not only neutrons, but also positrons. According to Irene and Frederick, the positrons in this nuclear reaction could not have been formed as a result of the formation of electron-positron pairs, but had to come from the atomic nucleus.
The Seventh Solvay Conference (5) took place in Brussels on October 22-29, 1933. It was called "The Structure and Properties of Atomic Nuclei". It was attended by 41 physicists, including the most prominent experts in this field in the world. Joliot reported the results of their experiments, stating that irradiating boron and aluminum with alpha rays produces either a neutron with a positron or a proton.. At this conference Lisa Meitner She said that in the same experiments with aluminum and fluorine, she did not get the same result. In interpretation, she did not share the opinion of the couple from Paris about the nuclear nature of the origin of positrons. However, when she returned to work in Berlin, she again carried out these experiments and on November 18, in a letter to Joliot-Curie, she admitted that now, in her opinion, positrons do indeed appear from the nucleus.
In addition, this conference Francis Perrin, their peer and good friend from Paris, spoke out on the subject of positrons. From experiments it was known that they obtained a continuous spectrum of positrons, similar to the spectrum of beta particles in natural radioactive decay. Further analysis of the energies of positrons and neutrons Perrin came to the conclusion that two emissions should be distinguished here: first, the emission of neutrons, accompanied by the formation of an unstable nucleus, and then the emission of positrons from this nucleus.
After the conference Joliot stopped these experiments for about two months. And then, in December 1933, Perrin published his opinion on the matter. At the same time, also in December Enrico Fermi proposed the theory of beta decay. This served as a theoretical basis for the interpretation of experiences. In early 1934, the couple from the French capital resumed their experiments.
Exactly on January 11, Thursday afternoon, Frédéric Joliot took aluminum foil and bombarded it with alpha particles for 10 minutes. For the first time, he used a Geiger-Muller counter for detection, and not the fog chamber, as before. He noticed with surprise that as he removed the source of alpha particles from the foil, the counting of positrons did not stop, the counters continued to show them, only their number decreased exponentially. He determined the half-life to be 3 minutes and 15 seconds. Then he reduced the energy of the alpha particles falling on the foil by placing a lead brake in their path. And it got fewer positrons, but the half-life didn't change.
Then he subjected boron and magnesium to the same experiments, and obtained half-lives in these experiments of 14 minutes and 2,5 minutes, respectively. Subsequently, such experiments were carried out with hydrogen, lithium, carbon, beryllium, nitrogen, oxygen, fluorine, sodium, calcium, nickel and silver - but he did not observe a similar phenomenon as for aluminum, boron and magnesium. The Geiger-Muller counter does not distinguish between positive and negative charged particles, so Frédéric Joliot also verified that it actually deals with positive electrons. The technical aspect was also important in this experiment, i.e., the presence of a strong source of alpha particles and the use of a sensitive charged particle counter, such as a Geiger-Muller counter.
As previously explained by the Joliot-Curie pair, positrons and neutrons are released simultaneously in the observed nuclear transformation. Now, following Francis Perrin's suggestions and reading Fermi's considerations, the couple concluded that the first nuclear reaction produced an unstable nucleus and a neutron, followed by beta plus decay of that unstable nucleus. So they could write the following reactions:
The Joliots noticed that the resulting radioactive isotopes had too short half-lives to exist in nature. They announced their results on January 15, 1934, in an article entitled "A New Type of Radioactivity". In early February, they succeeded in identifying phosphorus and nitrogen from the first two reactions from the collected small quantities. Soon there was a prophecy that more radioactive isotopes could be produced in nuclear bombardment reactions, also with the help of protons, deuterons and neutrons. In March, Enrico Fermi made a bet that such reactions would soon be carried out using neutrons. He soon won the bet himself.
Irena and Frederick were awarded the Nobel Prize in Chemistry in 1935 for "the synthesis of new radioactive elements". This discovery paved the way for the production of artificially radioactive isotopes, which have found many important and valuable applications in basic research, medicine, and industry.
Finally, it is worth mentioning physicists from the USA, Ernest Lawrence with colleagues from Berkeley and researchers from Pasadena, among whom was a Pole who was on an internship Andrei Sultan. The counting of pulses by the counters was observed, although the accelerator had already stopped working. They didn't like this count. However, they did not realize that they were dealing with an important new phenomenon and that they simply lacked the discovery of artificial radioactivity ...
