Natural pH Indicators
Under the influence of changes in the reaction of the environment, not only the compounds used in laboratories as indicators acquire different colors. An equally numerous group is made up of substances contained in natural products. In several trials, we will test the behavior of pH indicators in our environment.
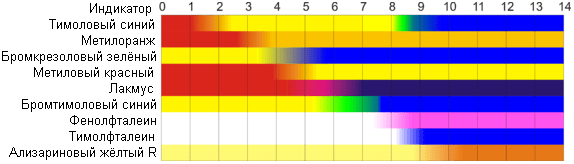
For experiments, several solutions with different pH will be required. They can be obtained by diluting hydrochloric acid with HCl (pH 3-4% solution is 0) and sodium hydroxide solution NaOH (4% solution has a pH of 14). Distilled water, which we will also use, has a pH of 7 (neutral). In the study, we will use beetroot juice, red cabbage juice, blueberry juice and tea infusion.
In test tubes with prepared solutions and distilled water, drop a little red beet juice (photo 1). In acidic solutions, it acquires an intense red color, in neutral and alkaline solutions, the color becomes brown, turning into a yellow tint (photo 2). The last color is the result of the decomposition of the dye in a strongly alkaline environment. The substance responsible for the discoloration of beetroot juice is betanin. Acidification of borscht or beetroot salad is a culinary “chip” that gives the dish an appetizing color.
In the same way, try red cabbage juice (photo 3). In an acid solution, the juice becomes bright red, in a neutral solution it becomes light purple, and in an alkaline solution it becomes green. Also in this case, the strong base decomposes the dye - the liquid in the test tube becomes yellow (photo 4). Substances that change color are anthocyanins. Sprinkling red cabbage salad with lemon juice gives it an appealing look.
Another experiment requires blueberry juice (photo 5). The red-violet color changes to red in an acidic medium, to green in an alkaline medium, and to yellow in a strongly alkaline medium (dye decomposition) (photo 6). Here, too, anthocyanins are responsible for changing the color of the juice.
Tea infusion can also be used as a solution pH indicator (photo 7). In the presence of acids, the color becomes straw yellow, in a neutral medium it becomes light brown, and in an alkaline medium it becomes dark brown (photo 8). Tannin derivatives are responsible for changing the color of the infusion, giving the tea its characteristic tart taste. The addition of lemon juice makes the color of the infusion lighter.
It is also worthwhile to independently conduct tests with other natural indicators - many juices and decoctions of plants change color due to acidification or alkalization of the environment.
See it on video:
Natural pH Indicators
