
How does galvanizing a body protect a car from corrosion?
Content
A car exists as long as it has a body. All other units are attached to its base and can be replaced with varying degrees of material costs. Yes, and the VIN number of the vehicle is located on the most tenacious parts welded into the overall structure. You can destroy the body in a serious accident or simply leaving it without protection against corrosion. Therefore, special attention is paid to the means of countering this harmful phenomenon.

What is galvanizing
A generally recognized effective way to put a rust barrier has been the use of zinc, in other words, galvanizing steel parts.
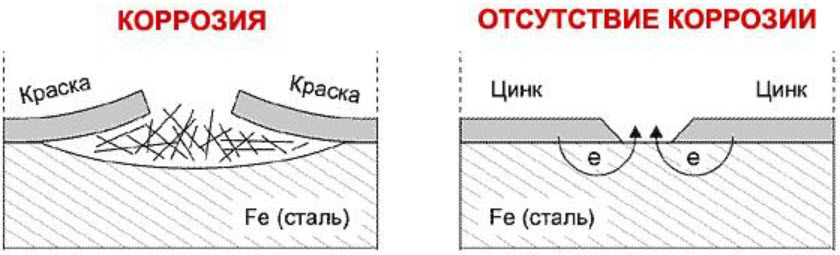
This method of protection contains two main aspects:
- the presence of a zinc coating on the body elements protects the base metal from the access of oxygen and water, which are the main enemies of iron, if it is not there in the form of a stainless alloy;
- zinc forms a galvanic pair with iron, in which, when water appears, it is zinc that begins to be consumed, unlike some other covering metals, on the contrary, accelerating the destruction of the base.
At the same time, zinc is relatively inexpensive, and the processes of its application are technologically well developed.
Advantages and disadvantages
Zinc coating is recognized by the automotive community as the best protection for body iron at an affordable price. When used together with a high-quality paintwork (LKP), this method has good advantages:
- good adhesion to the base metal, zinc itself does not exfoliate due to contact at the atomic level;
- the presence of double protection, both sealing and galvanic;
- the resistance of zinc itself to chemical wear, since it belongs to the category of metals capable of creating an impermeable oxide film on the surface, while not working as a catalyst for further corrosion;
- variety of application technologies;
- relative cheapness of protective metal.

There are disadvantages:
- although not significantly, the price of the body is still rising;
- the coating is not resistant to mechanical damage, in particular, it is destroyed during repair work on the body;
- the technological process is complicated in relation to environmental protection, zinc compounds are toxic;
- it is practically impossible in this way to provide reliable protection of welds and other joints of body parts.
Galvanization is carried out both in full and in part of the body, taking into account the threats from corrosion of the most susceptible parts, especially in the lower part of the car.
Types of car body galvanizing
The desire to reduce the cost of technological processes is forcing automakers to use methods of applying zinc that are different in efficiency.
Covering a car with zinc completely, and even in the most reliable way, few companies can afford. Such a car will be resistant to corrosion, but most likely it will not sell well due to the high price.
Hot
The highest quality coating method. During the production process, the part is completely immersed in molten zinc, after which a fairly thick layer remains on the surface, reliably bonded to iron.

Such protection is durable, reliable, and due to the large amount of tread, it lasts a long time and is able to even partially tighten minor mechanical damage.
The coating lasts 10 years or more, which allows the manufacturer to provide a long-term guarantee against through damage.
Electroplating
Zinc is applied to parts by electroplating in a special electrochemical bath. Atoms are transported by an electric field and firmly cling to the surface.

At the same time, the parts heat up less and the base metal does not lose its mechanical properties. The method requires the presence of a galvanic section harmful to the environment and consumes a significant amount of electricity.
Cold
A special powder is mixed into the primer applied to the body by spraying a fine zinc powder held on the surface by a primer layer.

The effectiveness is rather doubtful, since the galvanic pair of metals required for effective protection is almost not formed. Nevertheless, such protection gives some effect and is actively used. Providing more of an advertising effect than real protection against corrosion.
Zincmetal
The method is similar to the previous one, the coating includes two layers of protection from corrosion inhibitors, oxides and zinc powder. Differs in elasticity that promotes firmness in the course of production of the car.
The quality of protection is higher than that of cold galvanizing, but does not reach the efficiency of hot and galvanic methods. Technologies for the production of zinc metal can be different, sometimes heating and melting of the applied components are used.
Table of galvanizing car bodies of all brands
Huge volumes of production of makes and models of cars do not allow in a limited list to indicate specific methods of galvanizing bodies and the percentage of protected parts in the car.
But manufacturers apply the technology systematically, which makes it possible to roughly estimate the level of protection for individual brands in recent times.
| Car | Body galvanizing method | Level of protection by operating experience | Car price category | Service life of the body before corrosion |
| Audi | Hot single and double sided | Great | Premium | From 10 years |
| BMW | Electroplating | Good | Premium | From 8 years |
| Mercedes-Benz | Electroplating | Good | Premium | From 8 years |
| Volkswagen | Electroplating | Good | Business | From 8 years |
| Opel | Electroplating | Average | Standard | From 6 years |
| Toyota | Electroplating | Average | Standard | From 6 years |
| Hyundai | Cold | Inadequate | Standard | From 5 years |
| Volvo | hot full | Great | Business | From 10 years |
| Cadillac | hot full | Great | Premium | From 10 years |
| Daewoo | cold partial | Bad | Standard | From 3 years |
| Renault | Electroplating | Good | Standard | From 6 years |
| VAZ | Zinc metal | Satisfactory | Standard | From 5 years |
The service life of coatings can only be determined conditionally, since it strongly depends on the operating conditions.
In type testing, calibrated damage is applied to the bodywork, after which corrosion propagation is assessed in salt spray chambers, which are the worst conditions for body steel.
How to check if the car body is galvanized or not
This can be done by the research method, but it is expensive, it requires special equipment and partial destruction of the coatings. Therefore, the best way is to refer to the factory documentation for a specific model and operating experience from online reviews.
There are Internet resources where for each model you can get comprehensive information.
A factory warranty for the absence of through damage can also tell a lot. Typically, a period of about 12 years indicates a high-quality zinc coating.

For used cars, a lot of information carries the safety of iron in places where the paintwork has peeled off. High-quality galvanizing does not allow rust to grow even in the absence of varnish, paint and primer.
How to galvanize the body with a battery
Ordinary household batteries may contain a zinc cup, which plays the role of one of the electrodes. The shape of this part is convenient enough to create the simplest fixture for galvanizing. The car battery is used as a current source.
A cloth tampon is created around the zinc glass, which is impregnated with phosphoric acid. You can pre-dissolve a little zinc shavings prepared from the same battery in it. The plus of the battery is connected to the zinc, and the minus remains on the car body.
The place to be processed must be carefully cleaned mechanically from the slightest traces of rust. After that, the swab with zinc is pressed against the surface and the reaction begins to transfer zinc to body iron.
The process of coating formation can be observed visually. The resulting layer will be no worse than the one created in the galvanic bath of the plant.
At the end of the procedure, acid residues must be removed with a soda solution, the surface should be washed, dried and covered with technological layers of primer, paint and varnish.
