
Silicon-based cathodes stabilize Li-S cells. Effect: more than 2 charging cycles instead of several dozen
Scientists from the Daegu Institute of Science and Technology (DGIST, South Korea) have developed a silicon-based cathode that is expected to withstand more than 2 charge cycles in Li-S cells. Classic lithium-ion cells use pure silicon in the anodes to complement and gradually replace graphite. Silicon oxide was used here, and silicon dioxide was used in the cathode.
Li-S cell = lithium anode, silicon dioxide cathode with sulfur
Li-S cells are considered interesting because of their high energy density, weight and low manufacturing cost. However, no one has yet managed to create a version that would withstand more than several dozen charging cycles. All due to lithium polysulfides (LiPS), which dissolve in the electrolyte during discharge and react with the anode, reducing its capacity and, as a result, destroying the battery.
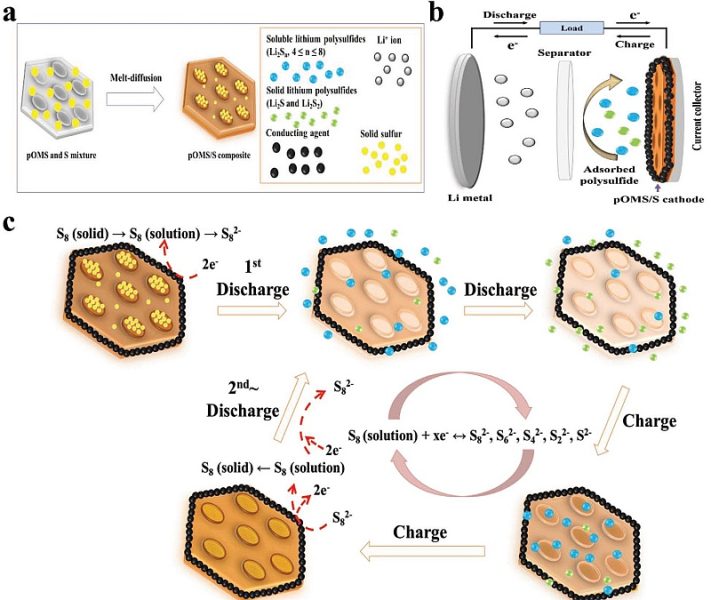
It is possible that South Korean researchers have found a solution to the problem. Instead of carbon-based materials (such as graphite), they used the cathode. lamellar structure of mesoporous silica (POMS).
The lamellar structure is understandable, while mesoporosity refers to the accumulation of pores (cavities) in silica that have a target size, areal density and small size dispersion (source). It is a bit like if you regularly poke through adjacent plates of some kind of silicate to make a sieve.
DGIST scientists used these holes to deposit sulfur in them (Figure a). During discharge, the sulfur dissolves and forms lithium polysulfides (LiPS) with lithium. Thus, the charge flows, but the LiPS remains trapped near the cathode due to the additional undefined carbon factor (black structure, figure b).
During charging, LiPS releases lithium, which is returned to the lithium anode. On the other hand, sulfur is converted to silica. No LiPS leakage to the anode, no metal damage.
The Li-S battery created in this way retains high capacity and stability for more than 2 working cycles. At least 500-700 cycles of operation are considered standard for classic Li-ion cells, although it should be added that well-processed lithium-ion cells can withstand several thousand cycles.
This may interest you: