
Nava: Our nanotube electrodes have 3 times the capacity and offer 10 times the power in lithium-ion cells.
New week and new battery. French supercapacitor manufacturer Nawa says it has begun manufacturing entirely new nanotube electrodes for lithium-ion batteries. It is assumed that due to the parallel arrangement of nanotubes, they can store three times more charge than carbon anodes.
Nawa's New 3D Anodes: Stronger, Better, Faster, Stronger
Modern lithium-ion anodes are mostly made using graphite or activated carbon (or even activated carbon from graphite), since their porous structure allows a large amount of ions to be stored. Sometimes carbon is mixed with silicon and surrounded by a nano-coating to limit swelling of the material.
You can already hear about fittings for using pure silicon, says Tesla or Samsung SDI.
> Completely new Tesla components: format 4680, silicon anode, “optimal diameter”, series production in 2022.
Nava says the structure of carbon is too complex for moving ions. Instead of carbon, the company wants to use carbon nanotubes, which are reportedly already used in the manufacturer's supercapacitors. Parallel nanotubes form vertical "notches" on which ions can settle comfortably. Literally:
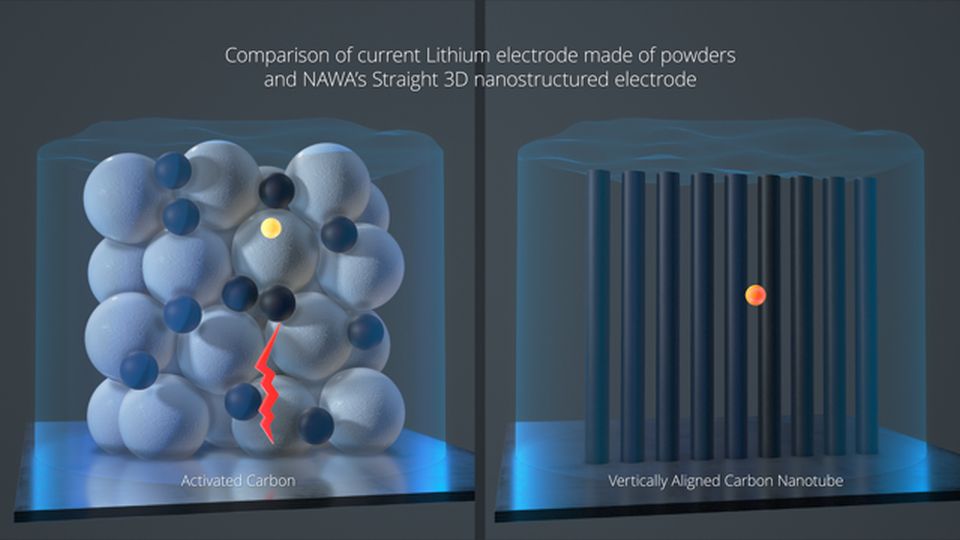
It can be assumed that all nanotubes in the anode are located in such a way that ions move freely between them until a convenient place is chosen. “Without wandering around the porous structures of a classical anode, the ions will only travel a few nanometers instead of micrometers, as is the case with classical electrodes,” Nava says.
The last statement shows that nanotubes can also act as cathodes - their function will depend on the material that will be on their surface. Nef doesn't rule out using silicon because the carbon nanotubes will encase it like a cage, so the structure won't have a chance to swell. Crush problem solved!
> Use off-the-shelf lithium-ion cells with a silicon anode. Charging faster than refueling with hydrogen
What would it be like with the parameters of cells using nanotubes? Well, they would allow:
- use 10 times more charging and discharging powerwhat now
- creation batteries with an energy density 2-3 times higher from contemporaries,
- extending battery life by five or even ten timesbecause nanotubes will not allow processes that destroy lithium-ion cells (source).
The very process of aligning nanotubes in a row should be trivially simple, allegedly the same mechanism that is used to coat glasses and photovoltaic cells with an anti-reflective coating. Nawa boasts that it can grow parallel nanotubes at speeds up to 100 micrometers (0,1 mm) per minute – and uses this technology in its supercapacitors.

If Nava's claims were true and the new electrodes went on sale, this would mean to us:
- electric vehicles are lighter than combustion vehicles, but with a longer range,
- the ability to charge electricians with a capacity of 500 ... 1 ... 000 kW, which is shorter than refueling,
- an increase in the mileage of electricians without the need to replace the battery from the current 300-600 thousand to 1,5-3-6 million kilometers,
- while maintaining the current size of the battery: rechargeable, say every two weeks.
Navah's first partner is the French battery manufacturer Saft, which partners with PSA Group and Renault in the European Battery Alliance.
Introductory photo: nanotubes in the Nawa (c) Nawa electrode
This may interest you:
