Reactions of mercury compounds
Metallic mercury and its compounds are highly toxic to living organisms. This is especially true for compounds that are highly soluble in water. Great care must be taken when experimenting with combinations of this unique element (mercury is the only metal that is liquid at room temperature). Compliance with the basic precepts of a chemist? will allow you to safely conduct several experiments with mercury compounds.
In the first experiment, we obtain aluminum amalgam (a solution of this metal in liquid mercury). Mercury (II) solution Hg nitrate (V) Hg (NO3)2 and a piece of aluminum wire (photo 1). An aluminum rod (carefully cleaned of deposits) is placed in a test tube with a solution of a soluble mercury salt (photo 2). After some time, we can observe the release of gas bubbles from the surface of the wire (photos 3 and 4). After removing the rod from the solution, it turns out that the clay is covered with a fluffy coating, and in addition, we also see balls of metallic mercury (photos 5 and 6).
Chemistry - the experience of combining mercury
Under normal conditions, the surface of aluminum is coated with a tightly fitting layer of aluminum oxide.2O3effectively isolates the metal from aggressive environmental influences. After cleaning and immersing the rod in a solution of mercury salt, Hg ions are displaced2+ more active aluminum
Mercury deposited on the surface of the rod forms an amalgam with aluminum, which makes it difficult for the oxide to adhere to it. Aluminum is a very active metal (it reacts with water to release hydrogen - gas bubbles are observed), and its use as a structural material is possible due to the dense oxide coating.
In the second experiment, we will detect ammonium NH ions.4+ using Nessler's reagent (the German chemist Julius Nessler was the first to use it in analysis in 1856).
Experiment on the reaction of hops and mercury compounds
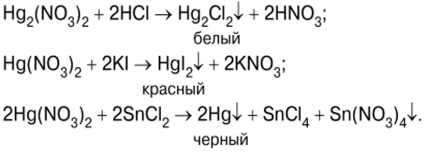
The test begins with the precipitation of mercury(II) iodide HgI.2, after mixing solutions of potassium iodide KI and mercury (II) nitrate (V) Hg (NO3)2 (photo 7):
Orange-red precipitate of HgI2 (photo 8) then treated with an excess of potassium iodide solution to obtain a soluble complex compound of formula K2HgI4 ? Potassium tetraiodercurate (II) (Photo 9), which is Nessler's reagent:
With the resulting compound, we can detect ammonium ions. Solutions of sodium hydroxide NaOH and ammonium chloride NH will still be required.4Cl (photo 10). After adding a small amount of ammonium salt solution to the Nessler reagent and alkalizing the medium with a strong base, we observe the formation of a yellow-orange color of the test tube contents. The current reaction can be written as:
The resulting mercury compound has a complex structure:
The highly sensitive Nessler test is used to detect even traces of ammonium salts or ammonia in water (eg tap water).

