Boiling, burning and flash point of gasoline
Content
What is gas?
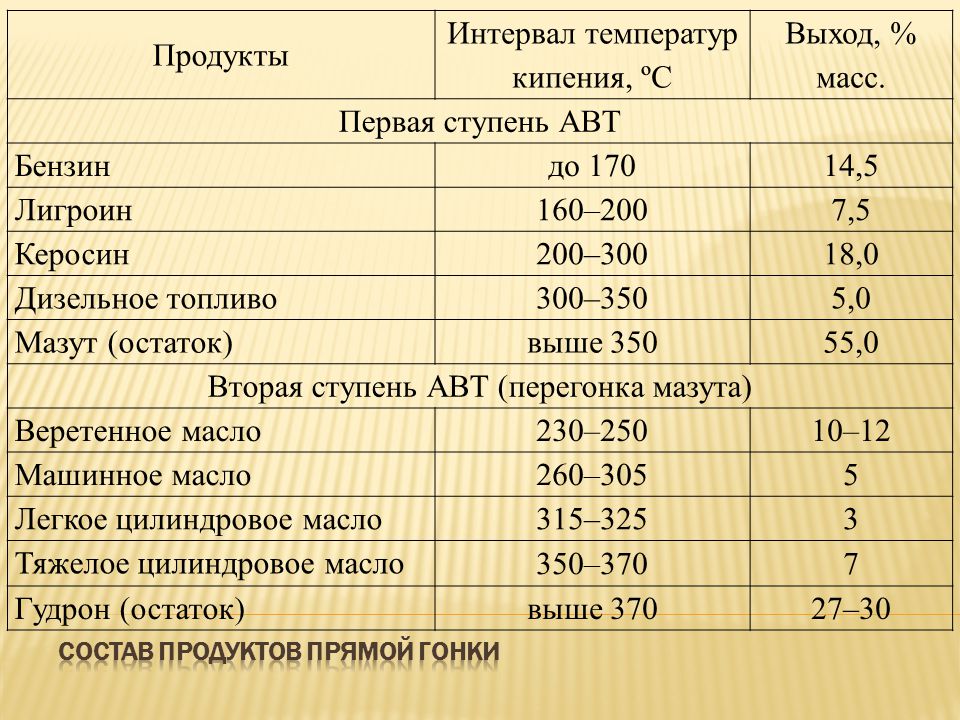
This point comes first because it is essential to understanding the issue. Looking ahead, let's say this: you will never find the chemical formula of gasoline. How, for example, you can easily find the formula of methane or another one-component petroleum product. Any source that will show you the formula of motor gasoline (it does not matter whether it is AI-76 that has gone out of circulation or AI-95, which is the most common now), is clearly mistaken.
The fact is that gasoline is a multicomponent liquid, in which at least a dozen different substances and even more of their derivatives are present. And that's just the base. The list of additives used in various gasolines, at different intervals and for various operating conditions, occupies an impressive list of several dozen positions. Therefore, it is impossible to express the composition of gasoline with a single chemical formula.


A brief definition of gasoline can be given as follows: a flammable mixture consisting of light fractions of various hydrocarbons.
Evaporation temperature of gasoline
The evaporation temperature is the thermal threshold at which spontaneous mixing of gasoline with air begins. This value cannot be unambiguously determined by one figure, as it depends on a large number of factors:
- the basic composition and additive package is the most significant factor that is regulated during production depending on the operating conditions of the internal combustion engine (climate, power system, compression ratio in the cylinders, etc.);
- atmospheric pressure - with increasing pressure, the evaporation temperature slightly decreases;
- way to study this value.

For gasoline, the evaporation temperature plays a special role. After all, it is on the principle of evaporation that the work of carburetor power systems is built. If gasoline stops evaporating, it will not be able to mix with air and enter the combustion chamber. In modern cars with direct injection, this characteristic has become less relevant. However, after injection of fuel into the cylinder by the injector, it is the volatility that determines how quickly and evenly the mist of small droplets mixes with the air. And the efficiency of the engine (its power and specific fuel consumption) depends on this.
The average evaporation temperature of gasoline is between 40 and 50°C. In the southern regions, this value is often higher. It is not controlled artificially, because there is no need for it. For the northern regions, on the contrary, it is underestimated. This is usually done not through additives, but through the formation of base gasoline from the lightest and most volatile fractions.


Boiling point of gasoline
The boiling point of gasoline is also an interesting value. Today, few young drivers know that at one time, in a hot climate, gasoline boiling in a fuel line or carburetor could immobilize a car. This phenomenon simply created traffic jams in the system. The light fractions were overheated and began to separate from the heavier ones in the form of combustible gas bubbles. The car cooled down, the gases became liquid again - and it was possible to continue the journey.
Сtoday, gasoline sold at gas stations will boil (with obvious bubbling with gas release) at about +80 ° C with a difference of + -30%, depending on the specific composition of a particular fuel.


Watch this video on YouTube
Flash point of gasoline
The flash point of gasoline is such a thermal threshold at which freely separated, lighter fractions of gasoline ignite from an open flame source when this source is located directly above the test sample.
In practice, the flash point is determined by the method of heating in an open crucible.
The test fuel is poured into a small open container. Then it is slowly heated up without involving an open flame (for example, on an electric stove). In parallel, the temperature is monitored in real time. Each time the temperature of gasoline rises by 1°C at a small height above its surface (so that an open flame does not come into contact with gasoline), a flame source is carried out. At the moment when the fire appears, and fix the flash point.
Simply put, the flash point marks the threshold at which the concentration of freely evaporating gasoline in the air reaches a value sufficient to ignite when exposed to an open fire.


Burning temperature of gasoline
This parameter determines the maximum temperature that burning gasoline creates. And here also you will not find unambiguous information that answers this question with one number.
Oddly enough, but it is for the combustion temperature that the main role is played by the conditions of the process, and not the composition of the fuel. If you look at the calorific value of various gasolines, then you will not see the difference between AI-92 and AI-100. In fact, the octane number determines only the resistance of the fuel to the appearance of detonation processes. And the quality of the fuel itself, and even more so the temperature of its combustion, does not affect in any way. By the way, often simple gasolines, such as AI-76 and AI-80, which have gone out of circulation, are cleaner and safer for humans than the same AI-98 modified with an impressive package of additives.


In the engine, the combustion temperature of gasoline is in the range from 900 to 1100°C. This is on average, with the proportion of air and fuel close to the stoichiometric ratio. The actual combustion temperature can either drop lower (for example, activating the USR valve somewhat reduces the thermal load on the cylinders) or increase under certain conditions.
The degree of compression also significantly affects the combustion temperature. The higher it is, the hotter it is in the cylinders.
Open flame gasoline burns at lower temperatures. Approximately, around 800-900 °C.


