Specific heat of combustion of kerosene
Content
The main thermophysical characteristics of kerosene
Kerosene is the middle distillate of the petroleum refining process, defined as the proportion of crude oil that boils between 145 and 300°C. Kerosene can be obtained from the distillation of crude oil (straight-run kerosene) or from the cracking of heavier oil streams (cracked kerosene).
Crude kerosene has properties that make it suitable for blending with various performance additives that determine its use in a variety of commercial applications, including transport fuels. Kerosene is a complex mixture of branched and straight chain compounds that can generally be divided into three classes: paraffins (55,2% by weight), naphthenes (40,9%) and aromatics (3,9%).


To be effective, all brands of kerosene must have the highest possible specific heat of combustion and specific heat capacity, and also be characterized by a fairly wide range of ignition temperatures. For various groups of kerosenes, these indicators are:
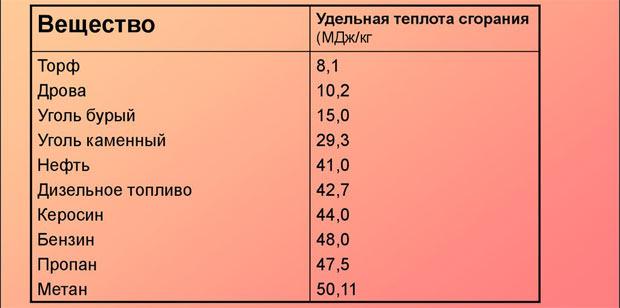
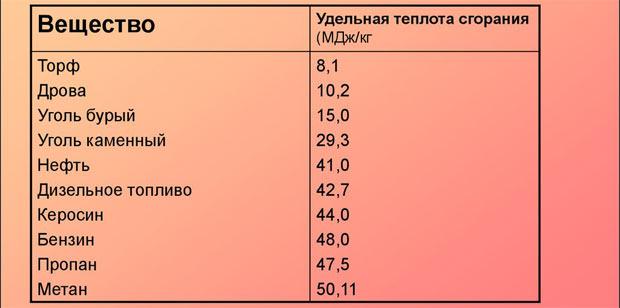
- Specific heat of combustion, kJ/kg — 43000±1000.
- autoignition temperature, 0C, not lower - 215.
- Specific heat capacity of kerosene at room temperature, J / kg K - 2000 ... 2020.
It is impossible to accurately determine most of the thermophysical parameters of kerosene, since the product itself does not have a constant chemical composition and is determined by the characteristics of the original oil. In addition, the density and viscosity of kerosene depends on external temperatures. It is only known that as the temperature approaches the zone of stable combustion of the oil product, the specific heat capacity of kerosene increases significantly: at 2000With it is already 2900 J / kg K, and at 2700C - 3260 J/kg K. Accordingly, the kinematic viscosity decreases. The combination of these parameters determines the good and stable ignition of kerosene.

The sequence of determining the specific heat of combustion
The specific calorific value of kerosene sets the conditions for its ignition in various devices - from engines to kerosene cutting machines. In the first case, the optimal combination of thermophysical parameters should be determined more carefully. Several schedules are usually set for each of the fuel combinations. These charts can be used to evaluate:
- The optimal ratio of the mixture of combustion products.
- The adiabatic temperature of the combustion reaction flame.
- Average molecular weight of combustion products.
- Specific heat ratio of combustion products.
This data is needed to determine the speed of the exhaust gases emitted from the engine, which in turn determines the thrust of the engine.


The optimal fuel mixture ratio gives the highest specific energy impulse and is a function of the pressure at which the engine will operate. An engine with high combustion chamber pressure and low exhaust pressure will have the highest optimum mixture ratio. In turn, the pressure in the combustion chamber and the energy intensity of kerosene fuel depend on the optimal mixture ratio.
In most designs of engines using kerosene as a fuel, much attention is paid to the conditions of adiabatic compression, when the pressure and volume occupied by the combustible mixture are in constant relationship - this affects the durability of the engine elements. In this case, as is known, there is no external heat exchange, which determines the maximum efficiency.


The specific heat capacity of kerosene is the amount of heat required to raise the temperature of one gram of a substance by one degree Celsius. The specific heat coefficient is the ratio of the specific heat at constant pressure to the specific heat at constant volume. The optimal ratio is set at a predetermined fuel pressure in the combustion chamber.
The exact indicators of heat during the combustion of kerosene are usually not established, since this oil product is a mixture of four hydrocarbons: dodecane (C12H26), tridecane (C13H28), tetradecane (C14H30) and pentadecane (C15H32). Even within the same batch of original oil, the percentage ratio of the listed components is not constant. Therefore, the thermophysical characteristics of kerosene are always calculated with known simplifications and assumptions.

