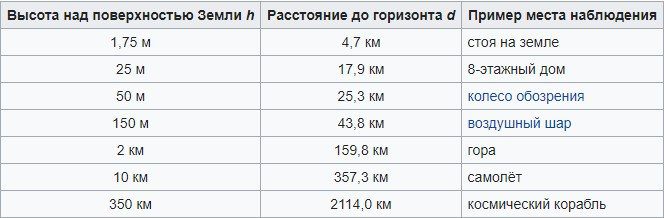
Air-to-air batteries provide a range of more than 1 km. Defect? They are disposable.
Content
A few days ago, we touched on the "inventive engineer," "father of eight," "navy veteran" who "invented batteries that used aluminum and a mysterious electrolyte." We found the development of the topic not very reliable - also thanks to the source, the Daily Mail - but the problem needs to be supplemented. If the British were dealing with aluminum-air batteries, then they ... really exist and can really offer a range of thousands of kilometers.
The inventor, described by the Daily Mail, "a father of eight," was presented as someone who has created something completely new (a non-toxic electrolyte) and is already in talks to sell his idea. Meanwhile, the topic of aluminum-air cells has been developed for several years.
But let's start from the very beginning:
Table of contents
- Aluminum Air Batteries - Live Fast, Die Young
- Tesla Model 3 Long Range with a power reserve of 1+ km? May be done
- Alcoa and Phinergy aluminum/air batteries - still disposable but well thought out
- Summary or why we criticized the Daily Mail
Aluminum-air batteries use the reaction of aluminum with oxygen and water molecules. In a chemical reaction (the formulas can be found on Wikipedia), aluminum hydroxide is formed, and eventually the metal bonds with oxygen to form alumina. The voltage drops rather quickly, and when all the metal has reacted, the cell stops working. Unlike lithium-ion batteries, Air-to-air cells cannot be recharged or reused..
They are disposable.
Yes, this is a problem, but cells have one very important feature: gigantic density of stored energy in relation to mass... This amounts to 8 kWh / kg. Meanwhile, the current level of the best lithium-ion cells is 0,3 kWh / kg.
Tesla Model 3 Long Range with a power reserve of 1+ km? May be done
Let's look at these numbers: 0,3 kWh/kg for the best modern lithium cells vs. 8 kWh/kg for aluminum cells - lithium is almost 27 times worse! Even if we take into account that in experiments, aluminum-air batteries reached a density of "only" 1,3 kWh / kg (source), this is still more than four times better than that of lithium cells!
So you don't need to be a great calculator to figure out that with Al-air Tesla Model 3 Long Range battery it will reach almost 1 km on battery instead of the current 730 km for lithium-ion... It's not much less than Warsaw to Rome, and less than Warsaw to Paris, Geneva or London!
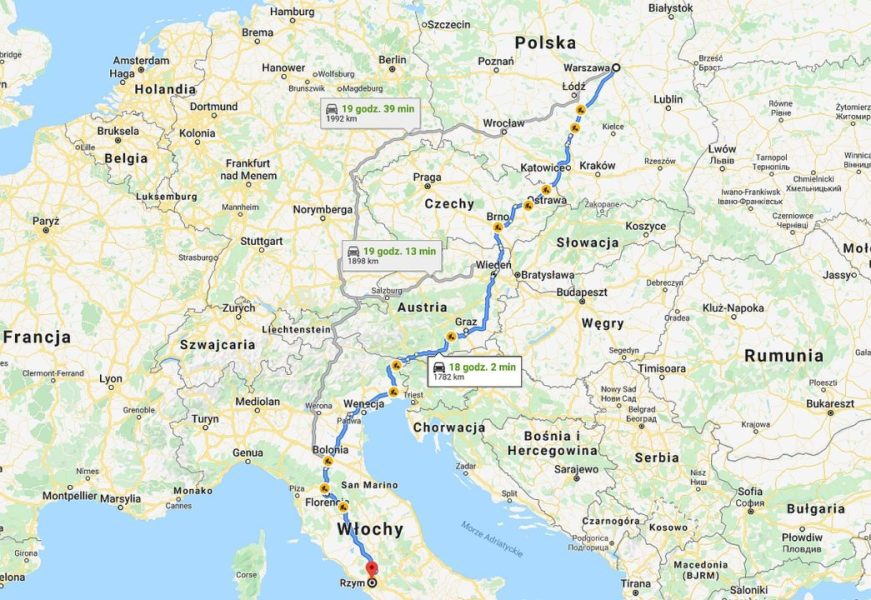
Unfortunately, with lithium-ion cells, after driving 500 kilometers with Tesla, we connect it to the charger for the time suggested by the car and move on. When using Al-air cells, the driver will have to go to a station where the battery will need to be replaced. Or its individual modules.
And while aluminum is cheap as an element, having to cook the element from scratch every time effectively negates the gains from the higher ranges. Corrosion of aluminum is also a problem that occurs even when the battery is not in use, but this problem has been solved by keeping the electrolyte in a separate container and pumping it when an aluminum-air battery is required.
Phinergy came up with this:
Alcoa and Phinergy aluminum/air batteries - still disposable but well thought out
Air batteries are ready to use commercial well, they are even used in military applications. They were created by Alcoa in partnership with Phinergy. In these systems, the electrolyte is in a separate container, and the individual cells are plates (cartridges) inserted into their compartments from above. It looks like:
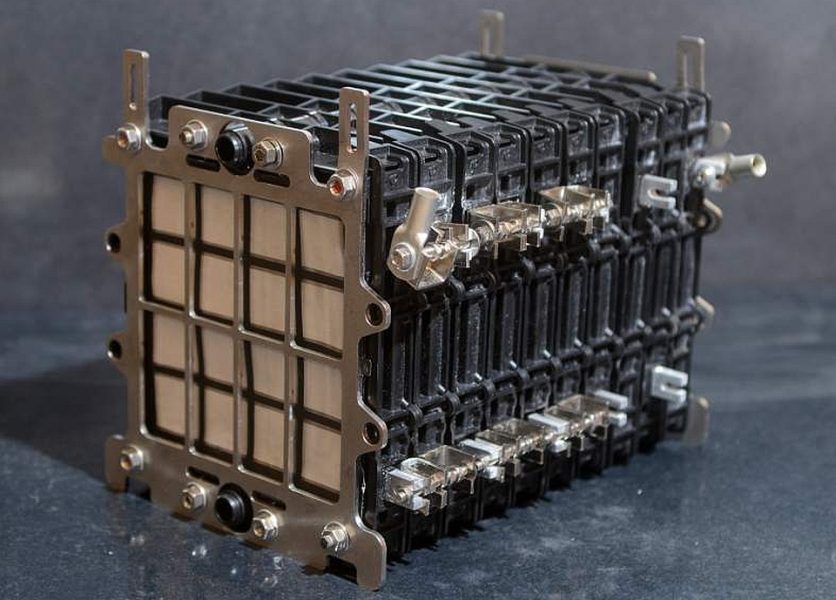
Aircraft battery (aluminum-air) of the Israeli company Alcoa. Note the tube on the side of the Alcoa electrolyte pump (c)
The battery is started by pumping electrolyte through the tubes (probably by gravity, since the battery acts as a backup). To charge the battery, you remove the used cartridges from the battery and insert new ones.
Thus, the owner of the machine will take the heavy system with him to use it one day if necessary. And when the need arises for charging, the car must be replaced by a person with the appropriate qualifications.
Compared to lithium-ion cells, the advantages of aluminum-air cells are lower production costs, no need for cobalt and reduced carbon dioxide emissions during production. The disadvantage is one-time use and the need to recycle used cartridges:
Summary or why we criticized the Daily Mail
Aluminum-air fuel cells (Al-air) already exist, are sometimes used, and have been worked on quite intensively in the last ten or so years. However, due to the increasing energy density of lithium-ion cells and the possibility of their repeated recharging, the topic has faded - especially in the automotive industry, where regularly replacing millions of batteries is a dizzying task..
We suspect that the inventor described by the Daily Mail probably did not invent anything, but constructed the aluminum-air cell himself. If, as he describes, he drank electrolyte in demonstrations, he must have used pure water for this purpose:
> The father of eight invented the 2 km battery? Mmm, yes, but no 🙂 [Daily Mail]
The biggest problem with aluminum-air batteries isn't that they don't exist - they do exist. The problem with them is one-time costs and high replacement costs. Investing in such a cell will sooner or later lose economic sense compared to lithium-ion batteries, because “charging” requires a visit to the workshop and a skilled worker.
There are about 22 million cars in Poland. According to the Central Statistical Office of Poland (GUS), we drive an average of 12,1 thousand kilometers per year. So, if we assume that the aluminum-air batteries will be replaced on average every 1 kilometer (for simplified calculation), each of these cars would have to visit the garage 210 times a year. Each of these cars visited the garage every 10 days on average.
603 cars are waiting for batteries EVERY DAY., also on Sundays! But such a replacement requires electrolyte suction, replacement of modules, checking all this. Someone will also have to collect these used modules from all over the country in order to process them later.
Now do you understand where our criticism came from?
Editorial Note www.elektrowoz.pl: The aforementioned Daily Mail article states that this is a "fuel cell" and not a "battery". However, to be honest, it should be added that “fuel cells "fall under the definition of" accumulator "valid in Poland. (see, for example, HERE). However, while an aluminum-air battery can (and should) be called a fuel cell, a lithium-ion battery cannot be called that.
A fuel cell works on the principle of externally supplied substances, often including oxygen, which reacts with another element to form a compound and release energy. Thus, the oxidation reaction is slower than combustion, but faster than normal corrosion. To reverse the process, a completely different type of device is often required.
On the other hand, in a lithium-ion battery, ions move between the electrodes, so there is no oxidation.
Note 2 to the www.elektrowoz.pl edition: the subtitle “live intense, die young” is taken from one of the studies on this topic. We like this because it describes the specifics of aluminum air cells.
This may interest you: