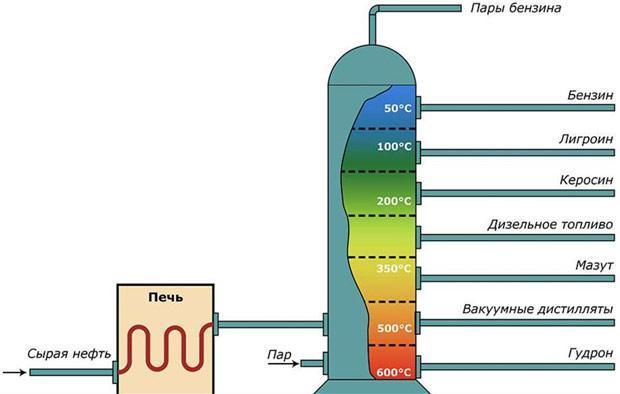
What is naphtha and where is it used?
Naphtha (less commonly called naphtha) is a highly volatile and flammable product of the distillation of crude oil. It finds use in many industries - both as a solvent and as a fuel. Naphtha exists in three forms - coal tar, shale, or oil. Each of these forms is formed under different conditions and is used according to its chemical properties.
Composition and characteristics
Depending on the duration of the formation of hydrocarbon substances, the composition naphtha different. For example, the "older" naphtha, which is based on oil, has a higher flash point, is less volatile and has a relatively high density. "Young" naphtha differs in opposite properties, and its basis is aromatic hydrocarbons.
The main physical properties of the product, therefore, are determined by the period of its primary formation. The most important ones are:
- Boiling temperature: 90...140ºС – for petroleum naphthas, and 60…80ºС – for aromatic naphthas (the latter, by the way, makes it difficult to determine them, since the same values are typical for petroleum ethers). Due to low boiling points naphthas are often referred to as petroleum spirits.
- Density: 750…860 kg/m3.
- Kinematic viscosity: 1,05…1,2 mm2/from.
- The temperature of the beginning of gelation is not higher: - 60ºС.
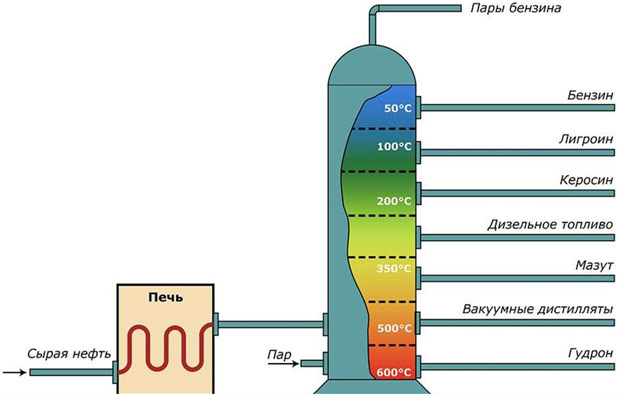

Naphtha does not dissolve in water and does not mix with it. The structural composition of naphthas includes hydrocarbons of the paraffinic and olefinic series, as well as naphthenic acids, and sulfur is present in a small amount of inorganic elements.
Where to use?
The use of naphtha is typical for the following purposes:
- Fuel for diesel engines.
- Solvent.
- Intermediate in the petrochemical industry.
Naphtha is used as a fuel because the product is flammable and is characterized by the release of a large amount of thermal energy upon ignition. The calorific value of naphtha reaches 3,14 MJ / l. Due to the fact that naphtha burns almost no soot, the product is often used in domestic and tourist heaters, lighting fixtures and lighters. Naphtha is rarely used directly as a fuel, due to its rather high toxicity; more often there are indications of the possibility of its use as an additive.


Enterprises for the production of such common plastics as polypropylene and polyethylene use naphtha as a raw material. Its derivatives are also widely used in the manufacture of butane and gasoline. Naphtha in these technologies is involved in the processes of steam cracking.
Naphtha as a solvent can be found in various cleaning products, where its low evaporation point is useful as a thinner for paints, varnishes and asphalt. The most well-known substances from this series are solvent and naphthalene. Due to its toxicity, naphtha is mainly used not for domestic purposes, but in enterprises (for example, those that dry-clean clothes).


Naphtha toxicity
Safety in the wide use of the considered oil product is limited by the following circumstances:
- High aggressiveness when exposed to the skin and cornea of the human eye. Upon contact with naphtha, the skin area swells painfully. It is recommended to wash the affected area with warm water as soon as possible.
- Nausea and damage to the lungs when swallowing even a small dose of the substance. This requires urgent hospitalization, otherwise respiratory failure occurs, which can lead to death.
- Strong specific odor (especially for "young" aromatic naphthas). Prolonged inhalation of vapors can cause breathing and mental problems. There is also information about the carcinogenicity of the substance.
Since the chemical is poisonous, it is strictly forbidden to drain its residues into uncontrolled containers (and, even more so, into open ones). It should also be remembered that ligroin is flammable and can cause a fire.


Watch this video on YouTube
