Operating a hydrogen vehicle (fuel cell)
Content

Another alternative for the operation of electric vehicles, hydrogen solution, has long been studied by the Germans and Japanese. Europe, which Tesla considers unstable, nevertheless decides to put a package on this technology (on a global scale, not for the sole purpose of propelling cars). So let's take a look at how the hydrogen car works, which is therefore only a variant of the electric car.
See also:
- Is a hydrogen car viable?
- What are the advantages and disadvantages of a fuel cell

Several types of hydrogen cars
While current technology is for cars that use fuel cells to power their electric motors, hydrogen can also be used in reciprocating internal combustion vehicles. It is indeed a gas that can be used in the same way as LPG and CNG already used in our vehicles. However, this idea was abandoned, the piston engine is really more in line with the time ...

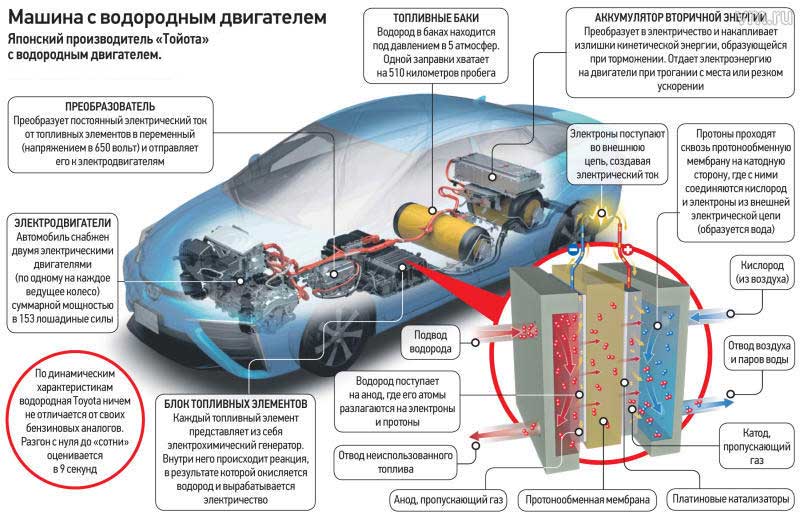
Here's a hydrogen-powered Toyota Mirai. It is sold in the USA, it is not in France, because there is no hydrogen distribution point there ... Having been late with the electrical terminals, we are already lagging behind in hydrogen!
Operating principle
If we had to summarize the system in one sentence, I would say thatit electric motor who walks with fuel non-polluting (in operation, not in production). Instead of charging the battery with a plug and therefore electricity, we fill it with liquid. This is why we call the fuel cell system (it is
accumulate
which works with fuel that
consumed
et
disappears from the tank
). In fact, the only difference with an electric motor is the storage of energy, here in a liquid, not a chemical form.
Therefore, it should be noted that the battery is discharging, unlike a lithium or even lead-acid battery (see the links to find out how they work).
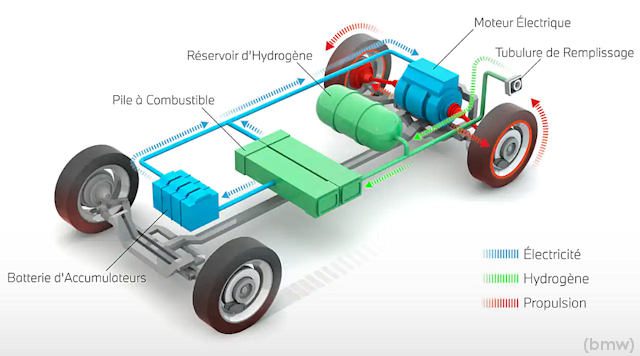
Process map
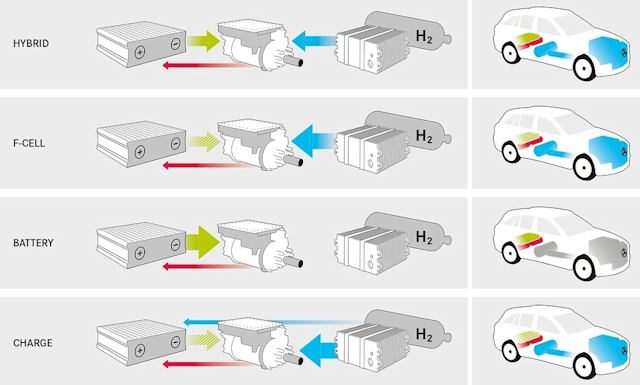
Hydrogen = hybrid?
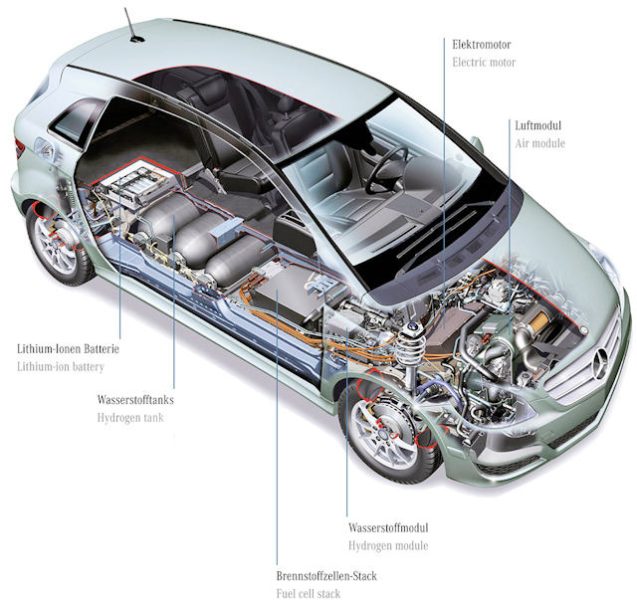
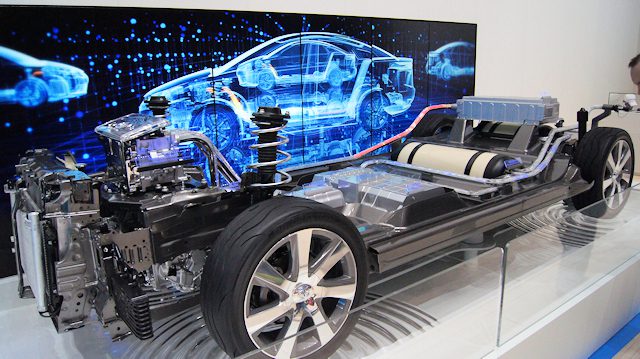
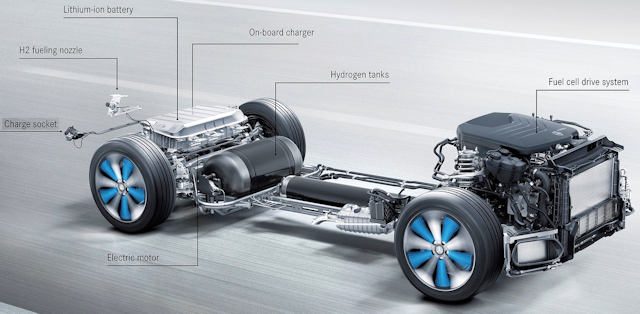
Almost ... Indeed, they systematically have an additional lithium battery, the usefulness of which I will explain below. Therefore, it is possible to operate only on hydrogen, only using a conventional battery, or even both at the same time.
Components
Hydrogen tank

We have a tank that can store 5 to 10 kg of hydrogen, knowing that each kilogram contains 33.3 kWh of energy (compared to electric vehicles, which have 35 to 100 kWh). The tank is specially designed and robust to withstand an internal pressure of 350 to 700 bar.
Fuel cell

The fuel cell will provide power to the car's electric motor, just like a conventional lithium battery. However, it needs fuel, namely hydrogen from the tank. It is made of very expensive platinum, but in the most modern versions it does without it.
Buffer battery

This is not required, but it is the standard for hydrogen vehicles. Indeed, it serves as a backup battery, a power amplifier (can work in parallel with a fuel cell), but also and above all, it serves to restore kinetic energy during deceleration and braking.
Power electronics
Not listed in my top diagram, the power electronics controls, interrupts and rectifies (converting between AC and DC currents) the various currents flowing through the various components of the car.

Refueling

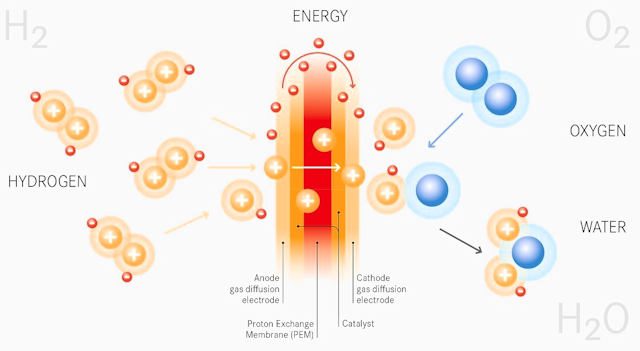
Fuel cell operation: catalysis

The goal is to extract electrons (electricity) from hydrogen in order to send them to an electric motor. This is all done through a controlled electrochemical reaction that separates electrons on one side (towards the engine) and protons on the other (in the fuel cell). The whole meeting ends up at the cathode, where the reaction ends: the final "mixture" gives water, which is pumped out of the system (exhaust).
Here is a diagram of catalysis, which is the extraction of electricity from hydrogen (reverse electrolysis).
Here we see the functioning of the fuel cell, namely the phenomenon of catalysis.
Hydrogen H2 (i.e. two hydrogen H atoms glued together: dihydrogen) goes from left to right. As it approaches the anode, it loses its nucleus (proton), which will be sucked down (due to the oxidation phenomenon). The electrons will then continue on their way to the right to subsequently use the electric motor.
In turn, we are reassembling everything by injecting O2 (oxygen from the air thanks to the compressor) on the cathode side, which will naturally allow the formation of a water molecule (which will catalyze all the elements into a single whole). a molecule that is a collection of Hs and Os).
Summary of chemical / physical reactions
ANODE : at the anode, the hydrogen atom is "cut" in half (H2 = 2e- + 2H+). The nucleus (H + ion) descends towards the cathode, while the electrons (e-) continue on their way due to their inability to pass through the electrolyte (the space between the anode and cathode).
CATHODE: at the cathode we see reverse (in different ways) ions H + and e- electrons. Then it is enough to introduce oxygen atoms so that all these elements want to collect, which then leads to the creation of a water molecule consisting of two hydrogen atoms and one oxygen atom. Or the formula: 2e- + 2H+ + O2 = H2O
Harvest ?
If we consider only the car itself, namely the efficiency of the tank to the end of the wheels (material transformation / mechanical reinforcement), we are here a little below 50%. Indeed, the battery has an efficiency of about 50%, and the electric motor - about 90%. Therefore, we first have 50% filtering, and then 10%.
If we take into account the efficiency of a power plant that generates energy, then before the production of hydrogen or even the distribution of electricity (in the case of lithium) we have 25% for hydrogen and 70% for electricity (approximately average, obviously).
Read more about profitability here.
Difference between a hydrogen car and a lithium battery electric car?
The cars are exactly the same, except for their "energy tank". Therefore, these are electric vehicles that use rotor-stator motors (induction, permanent magnets, or even reactive).

If a lithium battery also works thanks to a chemical reaction inside it (a reaction that naturally produces electricity: more precisely, electrons), nothing comes out of it, there is only an internal transformation. To return to its original state (recharging), it is enough to pass the current (connect to the sector) and the chemical reaction will start again in the opposite direction. The problem is that it takes time, even with superchargers.
For a hydrogen engine, which is a classic electric motor that is powered by a fuel cell (i.e. hydrogen), the battery consumes hydrogen during a chemical reaction. It is emptied through an exhaust that removes water vapor (the result of a chemical reaction).
Therefore, from a logical point of view, we could adapt any electric car to a hydrogen car, it is enough to replace the lithium battery with a fuel cell. So, in your understanding, the "hydrogen engine" should be considered primarily as an electric motor (see how it works here). He is necessarily approaching him, not because he is refueled as an entity.
The chemical reaction at the base of this tablet produces Heatof electricity (what we need for the electric motor) and water.

Why not everywhere?
The main technical problem with hydrogen is related to storage safety. In fact, like LPG, this fuel is dangerous because it becomes flammable on contact with air (and that's not all). So the problem is not only filling the car with fuel, but also having a tank strong enough to withstand any accident. Of course, the extra cost is also a big drag, and it seems less viable than a lithium-ion battery, which is dropping in cost dramatically.
Finally, the production and distribution network in the world is very underdeveloped, and governments want to produce hydrogen by electrolysis using renewable energy sources (many experts speak of a utopian scheme that cannot be realized in our "sudden" reality).
Ultimately, there is a better chance that conventional electricity will be the solution of choice for the future, rather than hydrogen, which will be used for a range of applications beyond individual mobility.
All comments and reactions
Dernier comment posted:
Bernard (Date: 2021, 09:23:14)
Hi,
Thank you for these strong and interesting ideas. I'll leave the site with a new firefly in my old brain.
Personally, I'm surprised that, apart from what I know about nuclear submarines, no one has developed a perfect engine for the road. It was indeed the one Philips unveiled at the 1971 Brussels Motor Show, with 200 hp. on two pistons.
Philips began operations in 1937-1938 and resumed in 1948.
In 1971, they claimed several hundred horsepower per piston. Since then I can't find anything ... Of course, Secret Defense.
What about gas turbine engines?
Your lanterns can add some water to my thinking mill.
Thanks for your knowledge and popularization.
Il I. 1 reaction (s) to this comment:
- Administrator SITE ADMINISTRATOR (2021-09-27 11:40:25): It's a lot of fun to read, thanks.
I don't know enough about this type of engine to judge, probably because of cost, size, difficult maintenance, average efficiency?
Bearing in mind that it is necessary to have a solution that allows the gas to be heated, and therefore its application on a regular public car is potentially dangerous (and that it will be constant over time).
In short, I suspect that you were hoping for a more accurate and confident answer ... Sorry.
(Your post will be visible under the comment after verification)
Write a comment
Using the electrical formula E, you will find that:

