How does a lithium ion battery for an electric vehicle work?
Having seen in another article the work of the lead battery that all cars are equipped with, let's now look at the principle of operation of an electric vehicle and especially its lithium battery ...

Князь
As with any type of battery, the principle remains the same: namely, to generate energy (here electricity) as a result of a chemical or even electrical reaction, because chemistry is always next to electricity. In fact, the atoms themselves are made of electricity: these are the electrons that revolve around the nucleus and which in some way form the “shell” of the atom, or even its “skin”. Knowing also that free electrons are flying pieces of skin that spend their time moving from one atom to another (without attaching to it), this is only in the case of conductive materials (depends on the number of layers of electrons and the number of electrons per last projectile).
We then take a "piece of skin" from atoms (hence some of its electricity) through a chemical reaction to produce electricity.

basics
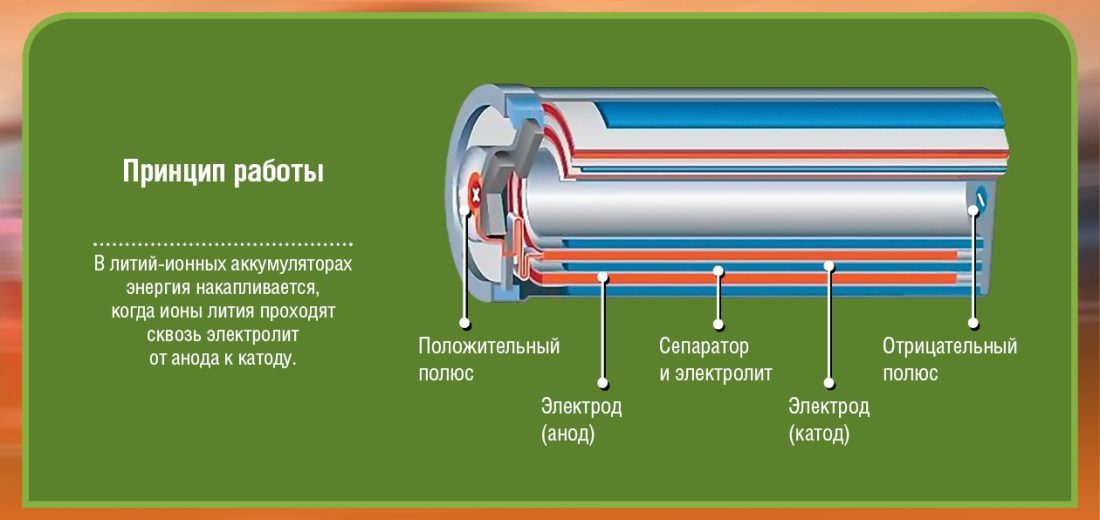
First of all, there are two poles (electrodes) which we call cathode (+ terminal: in lithium-cobalt oxide) and anode (terminal -: carbon). Each of these poles is made of material that either deflects electrons (-) or attracts (+). Everything is flooded electrolyte which will make possible a chemical reaction (transfer of material from the anode to the cathode) as a result of the generation of electricity. A barrier is inserted between these two electrodes (anode and cathode) to avoid short circuits.
Please note that the battery consists of several cells, each of which is formed by what is visible in the diagrams. If, for example, I accumulate 2 cells of 2 volts, I will have only 4 volts at the battery output. To set in motion a car weighing several hundred kg, imagine how many cells are needed ...
What is happening at the landfill?
On the right are lithium atoms. They are presented in detail, with the yellow heart representing the protons and the green heart representing the electrons they are orbiting.
When the battery is fully charged, all lithium atoms are on the anode (-) side. These atoms are made up of a nucleus (made up of several protons), which has a positive electrical force of 3, and electrons, to have a negative electrical force of 3 (1 in total, because 3 X 3 = 1). ... Therefore, the atom is stable with 3 positive and 3 negative (it does not attract or deflect electrons).
We detach an electron from lithium, which turns out to be with only two: then it is attracted to + and passes through the partition.
When I make contact between the + and - terminals (so when I use a battery), the electrons will move from the - terminal to the + terminal along the electrical wire external to the battery. However, these electrons come from the "hair" of lithium atoms! Basically, out of the 3 electrons spinning around, 1 is torn off and the atom only has 2 left. All of a sudden, its electrical force is no longer balanced, which also causes a chemical reaction. Note also that the lithium atom becomes lithium ion + because now it's positive (3 - 2 = 1 / The nucleus is worth 3 and the electrons are 2, since we lost one. Adding gives 1, not 0 as before. So it's no longer neutral).
The chemical reaction resulting from the imbalance (after breaking electrons to generate current) will result in the sending lithium ion + to the cathode (terminal +) through the wall designed to isolate everything. In the end, electrons and ions + end up on the + side.
At the end of the reaction, the battery is discharged. There is now a balance between the + and - terminals, which now prevents electricity. Basically, the principle is to induce depression on a chemical / electrical level in order to create an electrical current. We can think of this as a river, the more it slopes, the more important the intensity of the flowing water will be. On the other hand, if the river is flat, it will no longer flow, which means a dead battery.
Recharge?
Recharging consists of reversing the process by injecting electrons in a direction - and removing more by suction (it's a bit like replenishing the water of a river to use its flow again). Thus, everything in the battery is restored as it was before it was discharged.
Basically, when we discharge, we use a chemical reaction, and when we recharge, we return the original things (but for that you need energy and therefore a charging station).
Wear?
Lithium batteries wear out faster than the good old lead acid batteries that have been used in our cars for centuries. The electrolyte tends to decompose, like the electrodes (anode and cathode), but it should also be taken into account that a deposit forms on the electrodes, which reduces the transfer of ions from one side to the other ... Special devices allow you to recover used batteries by discharging them in a special way.
The number of possible cycles (discharge + full recharge) is estimated at about 1000-1500, so that with a half-cycle when recharging from 50 to 100% instead of 0 to 100%. HEATING also severely damages lithium-ion batteries, which tend to get hot when they draw too much power.
See also: How to save the battery in my electric car?
Engine power and battery ...
Unlike a thermal imager, power is not affected by the fuel tank. If you have a 400 hp engine, then having a 10 liter tank will not stop you getting 400 hp, even if it is for a very short time ... For an electric car, this is not the same at all! If the battery is not powerful enough, the engine will not be able to run at full capacity ... This is the case with some models where the engine can never be pushed to its limit (unless the owner fiddles and adds a large caliber battery!).
Now let's find out: how the ELECTRIC MOTOR works
All comments and reactions
Dernier comment posted:
mao (Date: 2021, 03:03:15)
very good work
Il I. 1 reaction (s) to this comment:
- Administrator SITE ADMINISTRATOR (2021-03-03 17:03:50): This comment is even better 😉
(Your post will be visible under the comment after verification)
Write a comment
How do you feel about the consumption figures declared by manufacturers?
