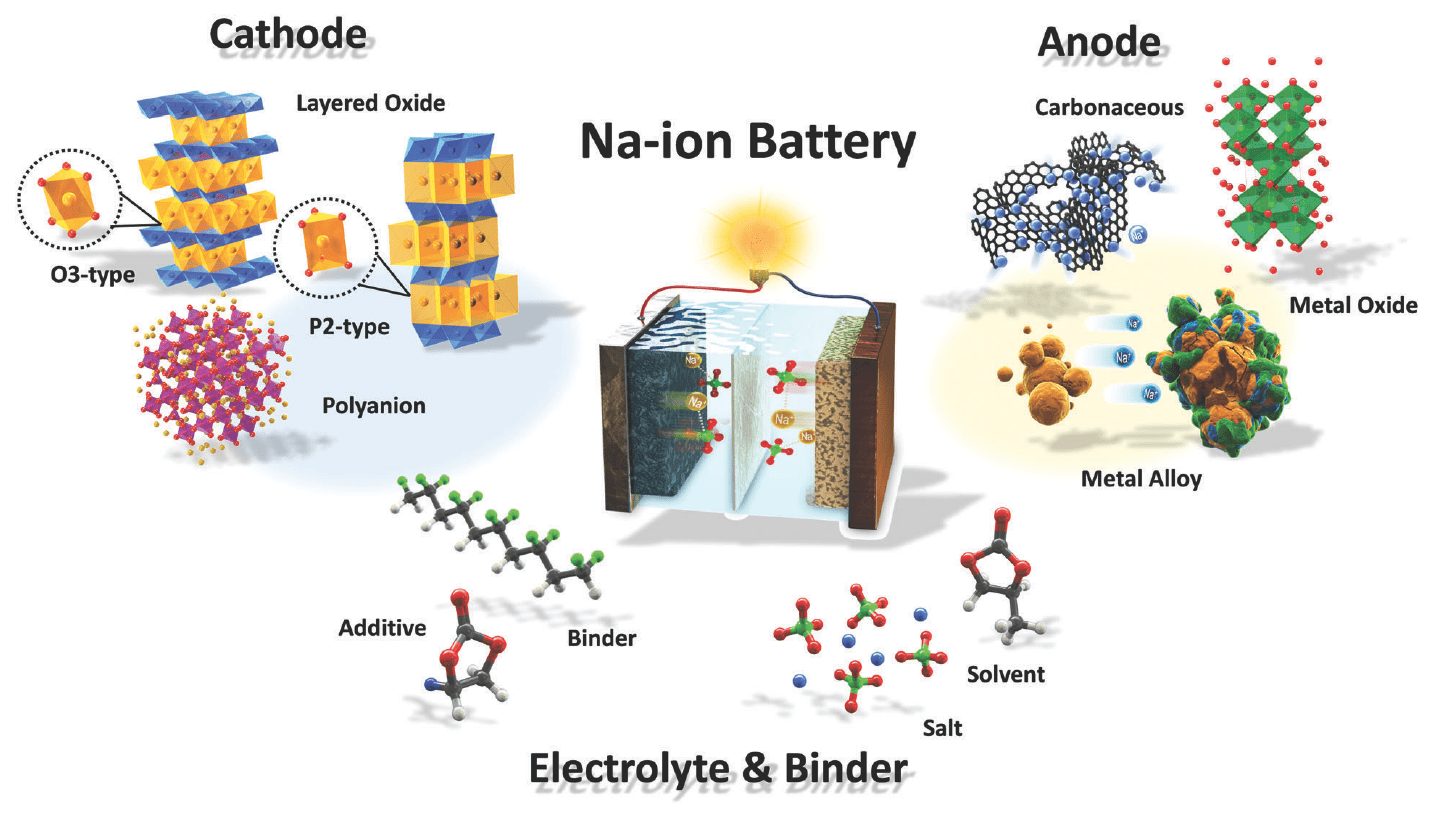
New week and new battery: Na-ion (sodium-ion), similar in parameters to Li-ion, but many times cheaper
Researchers at Washington State University (WSU) have created an "extra salt" battery that uses sodium instead of lithium. Sodium (Na) belongs to the group of alkali metals, has similar chemical properties, so cells based on it have a chance to compete with Li-ion. At least in some applications.
Na-ion batteries: much cheaper, slightly inferior to lithium-ion, at the research stage
Sodium is one of two elements in sodium chloride (NaCl) sodium chloride. Unlike lithium, it is found in abundance both in deposits (rock salt) and in the seas and oceans. Consequently, Na-ion cells can be many times cheaper than lithium-ion cells, and by the way, they must be designed using the same substances and structures as lithium-ion cells.
Work on Na-ion cells was carried out about 50-40 years ago, but was later discontinued. The sodium ion is larger than the lithium ion, so the elements have a problem keeping an appropriate charge. The structure of graphite - large enough for lithium ions - turned out to be too dense for sodium.
Research has revived in the past few years as the demand for reusable electrical components has skyrocketed. WSU scientists have created a sodium-ion battery that is supposed to store an amount of energy similar to that which can be stored in a similar lithium-ion battery. In addition, the battery lasted 1 charge cycle and retained over 000 percent of its original capacity (original).
Both of these parameters are considered "good" in the world of lithium-ion batteries. However, for elements with sodium ions, compliance with the conditions turned out to be difficult due to the growth of sodium crystals at the cathode. Therefore, it was decided to use a protective layer of metal oxide and electrolyte with dissolved sodium ions, which stabilized the structure. Succeeded.
The downside of a Na-ion cell is its lower energy density, which is understandable when you take into account the size of the lithium and sodium atoms. However, while this problem can be problematic in an electric vehicle, it does not completely affect energy storage. Even if Na-ion takes up twice as much space as lithium-ion, its price two or three times lower will make the choice obvious.
Only this is the earliest in a few years ...
This may interest you:
