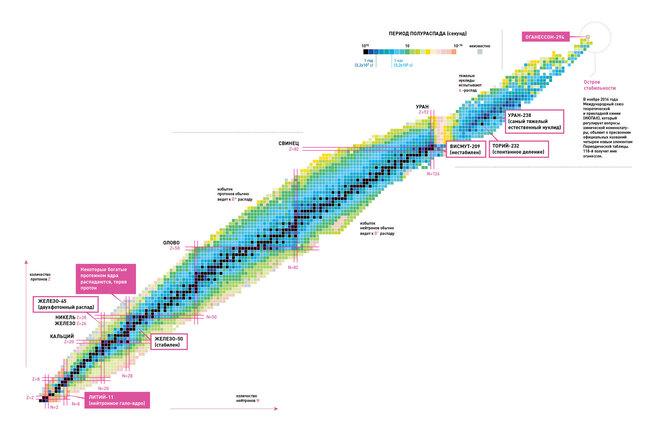
Limits of the periodic table of elements. Where is the happy island of stability?
Does the periodic table of elements have an "upper" limit - so is there a theoretical atomic number for a superheavy element that would be impossible to reach in the known physical world? Russian physicist Yuri Oganesyan, after whom element 118 is named, believes that such a limit must exist.
According to Oganesyan, head of the Flerov laboratory at the Joint Institute for Nuclear Research (JINR) in Dubna, Russia, the existence of such a limit is the result of relativistic effects. As the atomic number increases, the positive charge of the nucleus increases, and this, in turn, increases the speed of electrons around the nucleus, approaching the speed limit of light, the physicist explains in an interview published in the April issue of the journal. New Scientist. “For example, the electrons closest to the nucleus in element 112 travel at 7/10 the speed of light. If the outer electrons approached the speed of light, it would change the properties of the atom, violating the principles of the periodic table,” he says.
Creating new superheavy elements in physics laboratories is a tedious task. Scientists must, with the utmost precision, balance the forces of attraction and repulsion between elementary particles. What is needed is a "magic" number of protons and neutrons that "stick together" in the nucleus with the desired atomic number. The process itself accelerates the particles to a tenth of the speed of light. There is a small, but not zero, chance of the formation of a superheavy atomic nucleus of the required number. Then the task of physicists is to cool it as quickly as possible and “catch” it in the detector before it decays. However, for this it is necessary to obtain the appropriate "raw materials" - rare, extremely expensive isotopes of elements with the required neutron resources.
Essentially, the heavier an element in the transactinide group, the shorter its life. The element with atomic number 112 has a half-life of 29 seconds, 116 - 60 milliseconds, 118 - 0,9 milliseconds. It is believed that science reaches the limits of physically possible matter.
However, Oganesyan disagrees. He presents the point of view that he is in the world of superheavy elements. "Island of Stability". “The decay time of new elements is extremely short, but if you add neutrons to their nuclei, their lifetime will increase,” she notes. “Adding eight neutrons to elements numbered 110, 111, 112 and even 113 extends their life by 100 years. once".
Named after Oganesyan, the element Oganesson belongs to the group of transactinides and has atomic number 118. It was first synthesized in 2002 by a group of Russian and American scientists from the Joint Institute for Nuclear Research in Dubna. In December 2015, it was recognized as one of the four new elements by the IUPAC/IUPAP Joint Working Group (a group created by the International Union of Pure and Applied Chemistry and the International Union of Pure and Applied Physics). The official naming took place on November 28, 2016. Oganesson ma highest atomic number i largest atomic mass among all known elements. In 2002-2005, only four atoms of the 294 isotope were discovered.
This element belongs to the 18th group of the periodic table, i.e. noble gases (being its first artificial representative), however, it can show significant reactivity, unlike all other noble gases. In the past, oganesson was thought to be a gas under standard conditions, but current predictions point to a constant state of aggregation under these conditions due to the relativistic effects that Oganessian mentioned in the interview cited earlier. In the periodic table, it is in the p-block, being the last root of the seventh period.
Both Russian and American scholars have historically proposed different names for it. In the end, however, IUPAC decided to honor Hovhannisyan's memory by recognizing his great contribution to the discovery of the heaviest elements in the periodic table. This element is one of two (next to the seaborg) named after a living person.

