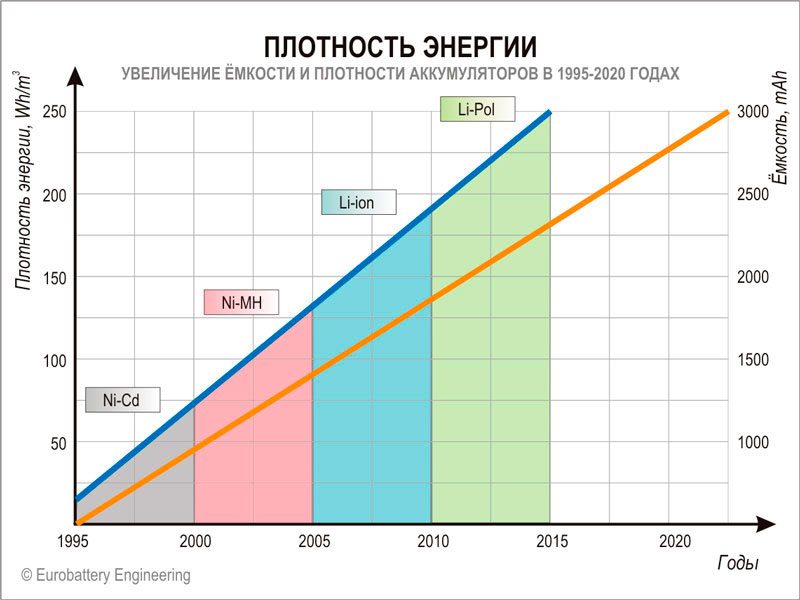
Stanford: We've reduced the weight of the lithium-ion pantographs by 80 percent. The energy density increases by 16-26 percent.
Scientists at Stanford University and the Stanford Linear Accelerator Center (SLAC) decided to shrink the lithium-ion cells to reduce their weight and thus increase the stored energy density. To do this, they reworked the load-bearing layers outward: instead of wide sheets of copper or aluminum, they used narrow strips of metal, supplemented with a layer of polymer.
Higher energy density in Li-ion without high investment costs
Each Li-ion cell is a roll consisting of a charge-discharge/discharge layer, an electrode, an electrolyte, an electrode, and a current collector in that order. The outer parts are metal foil made of copper or aluminum. They allow electrons to leave the cell and return to it.
Scientists from Stanford and SLAC decided to focus on collectors, because their weight is often several tens of percent of the weight of the entire link. Instead of copper sheets, they used polymer films with narrow copper strips. It turned out that it was possible to reduce the weight of the collectors by up to 80 percent:
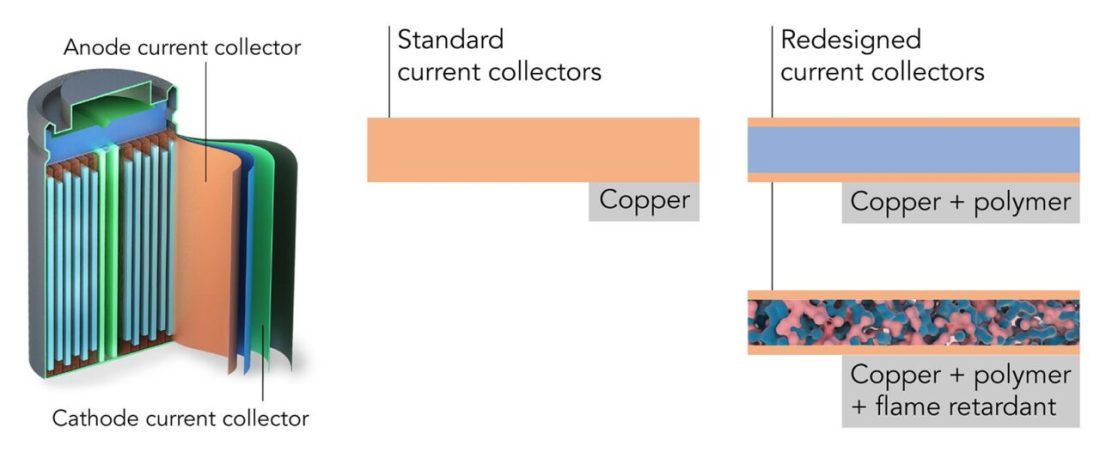
The classic cylindrical lithium-ion cell is a long roll consisting of several layers. Scientists from Stanford and SLAC have reduced the layers that collect charges and conduct them - current collectors. Instead of copper sheets, they used polymer-copper sheets enriched with non-flammable chemicals (c) Yusheng Ye / Stanford University
That's not all: chemical compounds can be added to the polymer that prevent the ignition, and then the lower flammability of the elements is accompanied by a lower weight:

Flammability of copper foil used in a classic lithium-ion cell and a collector developed by American researchers (c) Yusheng E / Stanford University
The researchers say recycled collectors can increase the gravimetric energy density of the cells by 16-26 percent (= 16-26 percent more energy for the same unit of mass). It means that a battery of the same size and energy density can be 20 percent lighter than current.
Attempts have been made in the past to optimize the reservoir, but changing them has led to unexpected side effects. The cells became unstable or more [expensive] electrolyte was required. The variant developed by scientists at Stanford does not appear to pose such problems.
These improvements are in early research, so don't expect them to hit the market before 2023. However, they look promising.
It should be added that Tesla also has an interesting idea to collect the charge of metal layers. Instead of using thin copper strips along the entire length of the roll and bringing them out in only one place (in the middle), it immediately brings them out using the overlapped cut edge. This makes the charges move a much smaller distance (resistance!), And copper provides additional heat transfer to the outside:
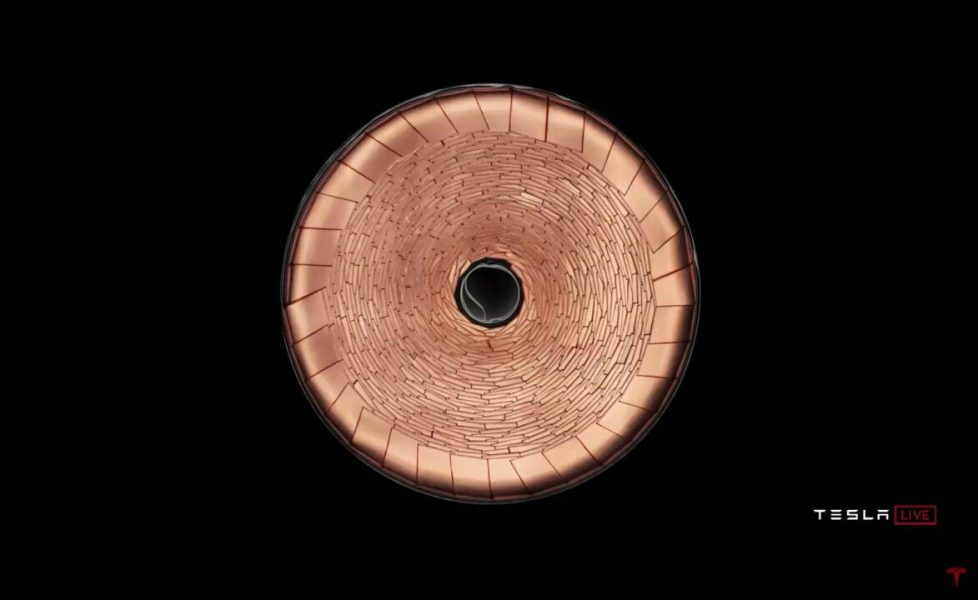
> Will the 4680 cells in Tesla's new batteries be cooled from the top and bottom? Only from below?
This may interest you:
