boiling point of brake fluid
Applied sense
The principle of operation of a modern brake system is based on the transmission of force from the pedal to the brake pads through hydraulics. The era of conventional mechanical brakes in passenger cars is long gone. Today, air or liquid acts as a carrier of energy. In passenger cars, in almost 100% of cases, the brakes are hydraulic.
Hydraulics as an energy carrier imposes some restrictions on the physical properties of the brake fluid.
Firstly, the brake fluid must be moderately aggressive towards other elements of the system and not cause sudden failures for this reason. Secondly, the liquid must tolerate high and low temperatures well. And thirdly, it must be absolutely incompressible.
In addition to these requirements, there are many others described in the FMVSS No. 116 standard of the US Department of Transportation. But now we will focus on only one thing: incompressibility.
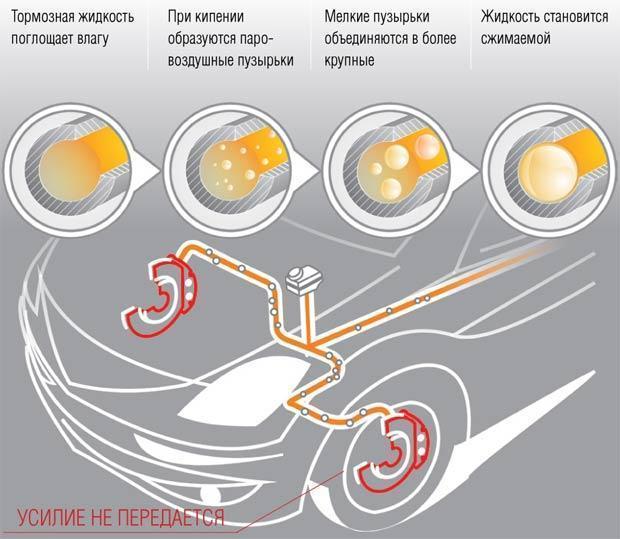

The fluid in the brake system is constantly exposed to heat. This happens when heat is transferred from heated pads and discs through the metal parts of the chassis of the car, as well as from internal fluid friction when moving through a system with high pressure. When a certain thermal threshold is reached, the liquid boils. A gas plug is formed, which, like any gas, is easily compressed.
One of the main requirements for the brake fluid is violated: it becomes compressible. The brakes fail, as a clear and complete transfer of energy from the pedal to the pads becomes impossible. Pressing the pedal simply compresses the gas plug. Almost no force is applied to the pads. Therefore, such a parameter as the boiling point of the brake fluid is given special attention.


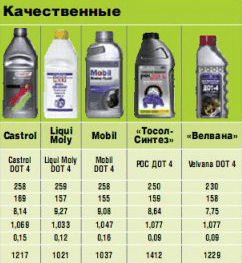
Boiling point of various brake fluids
Today, passenger cars use four classes of brake fluids: DOT-3, DOT-4, DOT-5.1 and DOT-5. The first three have a glycol or polyglycol base with the addition of a small percentage of other components that increase the performance of the fluid. Brake fluid DOT-5 is made on a silicone base. The boiling point of these liquids in their pure form from any manufacturer is not lower than the point indicated in the standard:
- DOT-3 - not less than 205°C;
- DOT-4 - not less than 230°C;
- DOT-5.1 - not less than 260°C;
- DOT-5 - not less than 260°C;
Glycols and polyglycols have one feature: these substances are hygroscopic. This means that they are able to accumulate moisture from the atmosphere in their volume. Moreover, water mixes well with glycol-based brake fluids and does not precipitate. This lowers the boiling point quite a lot. Moisture also adversely affects the freezing point of the brake fluid.
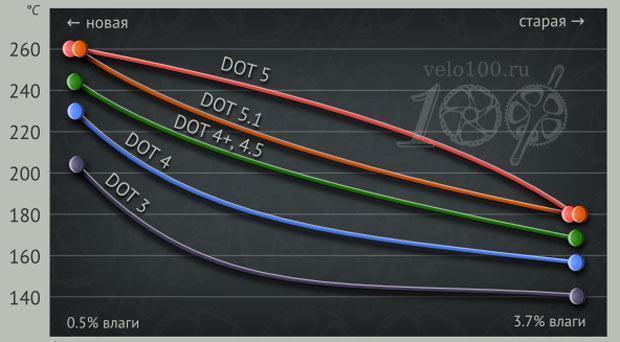

The following are generalized boiling point values for humidified liquids (with a water content of 3,5% of the total volume):
- DOT-3 - not less than 140°C;
- DOT-4 - not less than 155°C;
- DOT-5.1 - not less than 180°C.
Separately, you can highlight the silicone fluid class DOT-5. Despite the fact that moisture does not dissolve well in its volume and precipitates over time, water also lowers the boiling point. The standard notes the boiling point of a 3,5% moistened DOT-5 liquid at a level not lower than 180°C. As a rule, the real value of silicone fluids is much higher than the standard. And the rate of moisture accumulation in DOT-5 is less.
The service life of glycol liquids before the accumulation of a critical amount of moisture and an unacceptable decrease in the boiling point is from 2 to 3 years, for silicone liquids - about 5 years.


Watch this video on YouTube

